What is Ozone?-Ozone is a gas made up of three oxygen atoms (O3). It occurs naturally in small (trace) amounts in the upper atmosphere (the stratosphere). O + O3 → 2 O2
Chlorofluorocarbons and ozone
Many people have heard that chemicals called CFCs, short for chlorofluorocarbons, cause the ozone hole. CFCs escape into the atmosphere from 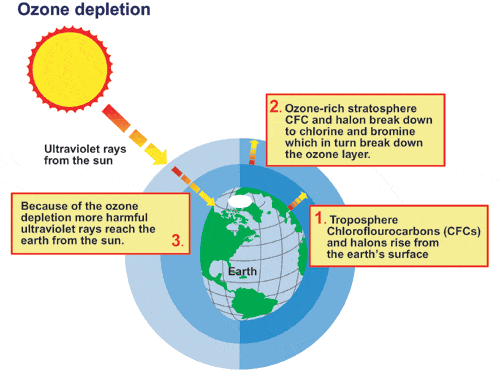 refrigeration and propellant devices and processes. In the lower atmosphere, they are so stable that they persist for years, even decades. This long lifetime allows some of the CFCs to eventually reach the stratosphere. In the stratosphere, ultraviolet light breaks the bond holding chlorine atoms (Cl) to the CFC molecule. A free chlorine atom goes on to participate in a series of chemical reactions that both destroy ozone and return the free chlorine atom to the atmosphere unchanged, where it can destroy more and more ozone molecules.
refrigeration and propellant devices and processes. In the lower atmosphere, they are so stable that they persist for years, even decades. This long lifetime allows some of the CFCs to eventually reach the stratosphere. In the stratosphere, ultraviolet light breaks the bond holding chlorine atoms (Cl) to the CFC molecule. A free chlorine atom goes on to participate in a series of chemical reactions that both destroy ozone and return the free chlorine atom to the atmosphere unchanged, where it can destroy more and more ozone molecules.
- Cl + O3 → ClO + O2 – The chlorine atom changes an ozone molecule to ordinary oxygen
- ClO + O3 → Cl + 2 O2 – The ClO from the previous reaction destroys a second ozone molecule and recreates the original chlorine atom, which can repeat the first reaction and continue to destroy ozone.
A Dobson Unit
The Dobson Unit is the most common unit for measuring ozone concentration. One Dobson Unit is the number of molecules of ozone that would 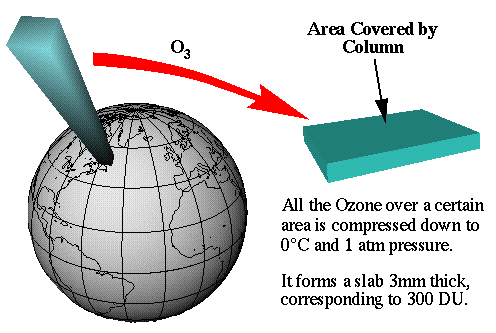 be required to create a layer of pure ozone 0.01 millimeters thick at a temperature of 0 degrees Celsius and a pressure of 1 atmosphere (the air pressure at the surface of the Earth). Expressed another way, a column of air with an ozone concentration of 1 Dobson Unit would contain about 2.69×1016 ozone molecules for every square centimeter of area at the base of the column. Over the Earth’s surface, the ozone layer’s average thickness is about 300 Dobson Units or a layer that is 3 millimeters thick.What scientists call the Antarctic Ozone “Hole” is an area where the ozone concentration drops to an average of about 100 Dobson Units, reduced by about 60% of their usual amount.
be required to create a layer of pure ozone 0.01 millimeters thick at a temperature of 0 degrees Celsius and a pressure of 1 atmosphere (the air pressure at the surface of the Earth). Expressed another way, a column of air with an ozone concentration of 1 Dobson Unit would contain about 2.69×1016 ozone molecules for every square centimeter of area at the base of the column. Over the Earth’s surface, the ozone layer’s average thickness is about 300 Dobson Units or a layer that is 3 millimeters thick.What scientists call the Antarctic Ozone “Hole” is an area where the ozone concentration drops to an average of about 100 Dobson Units, reduced by about 60% of their usual amount.
The best way is simply to let it repair itself. Over time, the natural ozone cycle will replenish the depleted mass to what we think of as “normal” levels, but only if there is no interference by ozone-depleting chemical compounds in the atmosphere. Unfortunately, this poses a serious problem, because due to the way that our atmosphere is layered, it will take around fifty years before the CFC’s and other ozone depleting chemicals to actually reach the stratospheric layer where the ozone exists, so even if we stopped their release, fright now, it will be fifty years before the ozone layer can even begin to fix itself.
