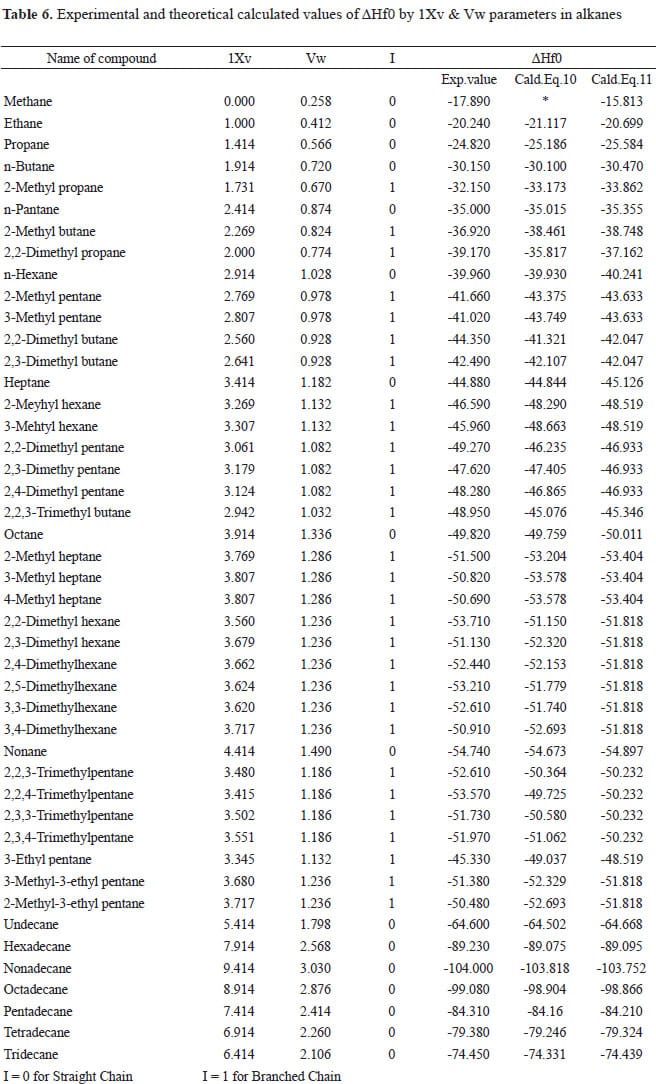
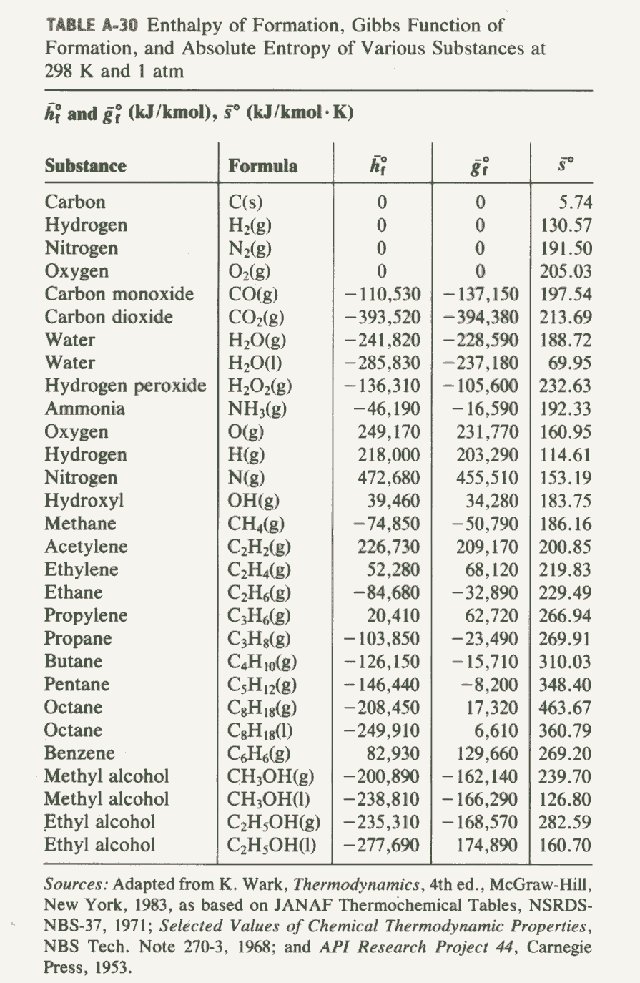
Standard Enthalpies of Formation, ΔH°f
The quantity of energy associated with the formation of one mole of a substance from its elements (in their standard states). The standard states (at SATP) of most elements are solid, except for the gaseous diatomic molecules, H2, O2, N2, F2, Cl2, and the two liquid elements Hg and Br2. These energies can be looked…