- Capillary action, or capillarity, is a phenomenon where liquid spontaneously rises in a narrow space such as a thin tube, or in porous materials such as paper or in some non-porous materials such as liquefied carbon fibre.
- This effect can cause liquids to flow against the force of gravity or the magnetic field induction.
- It occurs because of inter-molecular attractive forces between the liquid and solid surrounding surfaces
- If the diameter of the tube is sufficiently small, then the combination of surface tension (which is caused by cohesion within the liquid) and forces of adhesion between the liquid and container act to lift the liquid.
- Adhesion is the tendency of dissimilar particles and/or surfaces to cling to one another (cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another).
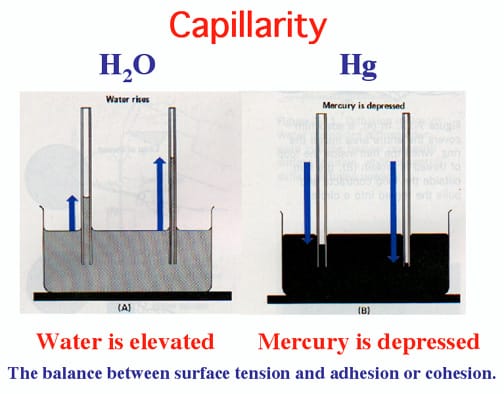
- Water forms interface with empty glass beaker; requires LESS energy than forming a surface with air
- Full liquid Mercury doesn’t form interface with glass; takes TOO MUCH energy
Tube with SMALLER diameter will have a GREATER height in water level when submerged! Less ENERGY needed to form a surface with air and interface with glass
Height of liquid in column: hliquid= (2 x ơliquid)/ (densitylquid x gravity) x (cos0)/ r
***Set cos0= 1 to solve for “max height”
Wetting
- Different liquid like to form interfaces differently with liquids
- If liquid forms an upward-curved meniscus- called wetting
- If liquid forms an downward-curved meniscus- called non-wetting
Mechanical Equilibrium for raindrop (Young-Dupre equation)
Ơglass/air= ơglass/liquid + ơliquid/air x cos0