Background
Osmosis is the movement of water molecules from a high-concentration region to a low-concentration region (Guyton and Hall, 2021). Only the free water molecules in the solution can move; the solute molecules remain stationary (Raven, Johnson, and Mason, 2014). Osmosis continues until the concentration of water molecules on either side of the membrane reaches equilibrium (Raven, Johnson, and Mason, 2014). Despite the difference in the number of water molecules and solute molecules in the two compartments, their concentration ratio remains the same across the membrane.
Once equilibrium is achieved, osmosis ceases, but random movement of particles from both sides of the membrane continues, maintaining the equal concentration levels (Raven, Johnson, and Mason, 2014). Osmosis occurs down a concentration gradient, a natural process that does not require external energy input (Science Sauce, 2017). A solution with a higher concentration of water molecules (more dilute) is termed hypotonic (BBC Bitesize, n.d.). Conversely, a solution with a lower concentration of water molecules (more concentrated solute) is termed hypertonic (BBC Bitesize, n.d.). When the concentration of water molecules is equal on both sides of the membrane, the solution is considered isotonic (Science Sauce, 2017).
Osmosis is crucial in plant cells (Taiz and Zeiger, 2015). When plant cells are exposed to a solution with a higher water concentration than their internal environment, water molecules move into the cells, causing the cytoplasm and cell membrane to press against the cell wall (Pickard, 2022). The cell wall serves as a protective barrier, preventing the cell from bursting under these conditions (Campbell et al., 2008). This state is referred to as cell turgor (Pickard, 2022). Conversely, suppose plant cells are placed in a solution with a lower water concentration than their internal environment. In that case, water molecules move out of the cells, leading to the vacuole’s shrinkage and the cell membrane’s separation from the cell wall (Campbell et al., 2008). This process is known as plasmolysis (Taiz and Zeiger, 2015).
The concentration of water molecules is an important factor in the osmosis rate. Suppose the difference in concentration of water molecules between either side of the plant membrane is very large. In that case, osmosis will occur at a faster rate because there is a steep concentration gradient. On the other hand, if the difference in concentration of water molecules on either side of the plant membrane is lower, osmosis will take place at a slower rate since there is a gentle concentration gradient. Another significant factor is temperature. At a greater temperature, water molecules gain heat energy which is converted into kinetic energy. This, therefore, results in a greater movement of particles and a quicker rate of reaction. Hence, temperature must be controlled throughout the experiment.
Hypothesis
It is hypothesised that as the concentration of the salt solution increases, the mass of a cylinder of a potato will decrease. This hypothesis is rooted in the principle of osmosis, where a higher salt concentration in the surrounding solution would prompt water molecules from the potato cells to diffuse outward, equalising the water concentration on both sides of the membrane. Consequently, plasmolysis of potato cells would occur, causing the membrane to detach from the cell wall and compress the organelles. This process is expected to reduce the potato cores’ mass and size as the salt concentration in the solution intensifies. Furthermore, it is hypothesised that a steeper concentration gradient will lead to a more significant reduction in the potato cores’ mass over time, illustrating a linear relationship between the percentage change in mass and the salt solution’s concentration. Conversely, when placed in distilled water without salt, the potato cores are expected to gain mass due to the higher concentration of water molecules in the solution compared to the potato cells.
Variables
| Independent Variable: | Concentration of salt solution | Using stock solution of 1M (58.44 g of salt to 1L of water), For 40cm3 of total solution (50cm3 made then measured out): 1M (50cm3 of 1M salt solution, 0cm3 of distilled water)0.8M (40cm3 of 1M salt solution, 10cm3 of distilled water)0.6M (30cm3 of 1M salt solution, 20cm3 of distilled water)0.4M (20cm3 of 1M salt solution, 30cm3 of distilled water)0.2M (10cm3 of 1M salt solution, 40cm3 of distilled water) |
| Dependent Variable: | Mass of weight of potato cylinder after soaking in salt solution | Measured in grams, before and after salt solution bath. |
| Controlled Variables: | Volume of water | 40cm3 |
| SA to weight ratio | ||
| Length of time the potato is left to soak in salt solution | 20 mins | |
| Temperature of water | Room temp |
Materials
- 1x Electronic balance
- 1x Weighing boat
- 5x Test tubes
- 1x Test tube rack
- 2x Measuring cylinders (20cm3)
- 100cm3 of Distilled Water
- 150cm3 of Salt Solution (1 mol/dm3)
- 1x Potato corer
- 1x Glass Rod
- 1x Scalpel
- 1x Tweezers
- 1x Tile
- 1x Potatoes
- Sufficient Paper Towels
- 1x Safety Goggles
- 1x Timing device
- 1x Ruler
Method
Collect all of the equipment detailed in the equipment list:
- Conduct this practical whilst standing to avoid getting wet in the event of a spillage. Put on a pair of safety goggles.
- Turn on your electronic balance. Check that it reads 0.00g after the weighing boat has been placed on top of it.
- Collect your potato and the potato corer. Carefully stick the corer into the potato and keep pushing until it comes out from the other side. Practise care to make sure that you do not hurt yourself.
- Using a glass rod, push the potato core out of the potato corer.
- Repeat steps 4-5, four more times, making sure not to intersect other holes made in the potato as this will cause uneven cylinders and may interfere with the results.
- Place all of the potato cores on a tile.
- Once you have 5 potato cores, cut off the skin from each of them individually using a scalpel. Keep your fingers away from the scalpel’s blade at all times when doing this.
- Using the ruler and the scalpel, cut the potato cores to 4cm in length, being as accurate as possible.
- Using the balance, weigh each core. Note that the mass should be equal in all cores.
- Using a measuring cylinder, prepare the salt solutions of different concentrations as follows and pour into different boiling tubes:
- 1M (50cm3 of 1M salt solution, 0cm3 of distilled water)
- 0.8M (40cm3 of 1M salt solution, 10cm3 of distilled water)
- 0.6M (30cm3 of 1M salt solution, 20cm3 of distilled water)
- 0.4M (20cm3 of 1M salt solution, 30cm3 of distilled water)
- 0.2M (10cm3 of 1M salt solution, 40cm3 of distilled water)
- Make sure to label the tubes as to not get mixed up when conducting the experiment
- Collect the timing device and set up the apparatus as shown in the visualisation above
- Start by using a salt solution of a concentration of 1 mol/dm3
- Carefully drop in the potato cores into the salt solutions using tweezers
- Start a timer for 20 minutes
- When the timer ends, using tweezers, pick up the potato core inside of the test tube and carefully release it onto a towel.
- Dry the potato core thoroughly.
- With the tweezers, put the potato core onto the weighing boat and record its mass
- Discard the potato core.
Safety
| Hazard | Risk | How danger will be avoided | Response if Hazard occurs |
| Sharp instruments (Scalpel) | Can result in severe cuts | Exercise extreme caution when handling the scalpel, a sharp and potentially dangerous tool. Maintain a safe distance between your fingers and the blade throughout its use. Focus intently on the cutting task at hand and refrain from recklessly waving the scalpel. When not in use, secure the scalpel by placing its cap back on or storing it in a designated safe area to prevent unintentional injuries. | In the event of an accidental scalpel cut, promptly wash the affected area, including your hands, under running water. Immediately inform a teacher or technician, especially if the cut appears severe. They will provide you with the necessary first aid supplies to clean and dress the wound. |
| The potato corer is used irresponsibly | The user of the potato corer could get stabbed | Employ the potato corer with caution, avoiding excessive force during the coring process. Ensure that the core is extracted from the centre of the potato and that the corer is inserted longitudinally, not laterally. Position your hands firmly on the sides of the potato to prevent accidental stabbing upon the corer’s emergence from the opposite end. | in the event of an accidental puncture or injury caused by the potato corer, promptly cleanse the affected area, including your hands, under running water. This is particularly crucial if any bleeding occurs. Immediately inform a teacher or technician, especially if bleeding is evident. Do not resume the experiment until the incident has been addressed. |
| Glass equipement is dropped | Pieces of glass can cause cuts and could go in the eyes | Maintain an upright stance throughout the experiment to enhance reaction time in case of accidental spills or drops. Refrain from placing glass equipment on the edge of the workstation to minimise the risk of breakage upon impact. To protect against eye injuries from flying glass fragments, safety goggles must be worn at all times. | In the event of dropped glassware, prioritise quick reaction and vacate the immediate vicinity. If any cuts occur, promptly inform a teacher or technician, as glass fragments may remain embedded in the tissue. Regardless of the situation, immediately notify a responsible adult supervisor. Refrain from attempting to collect the broken glass on your own |
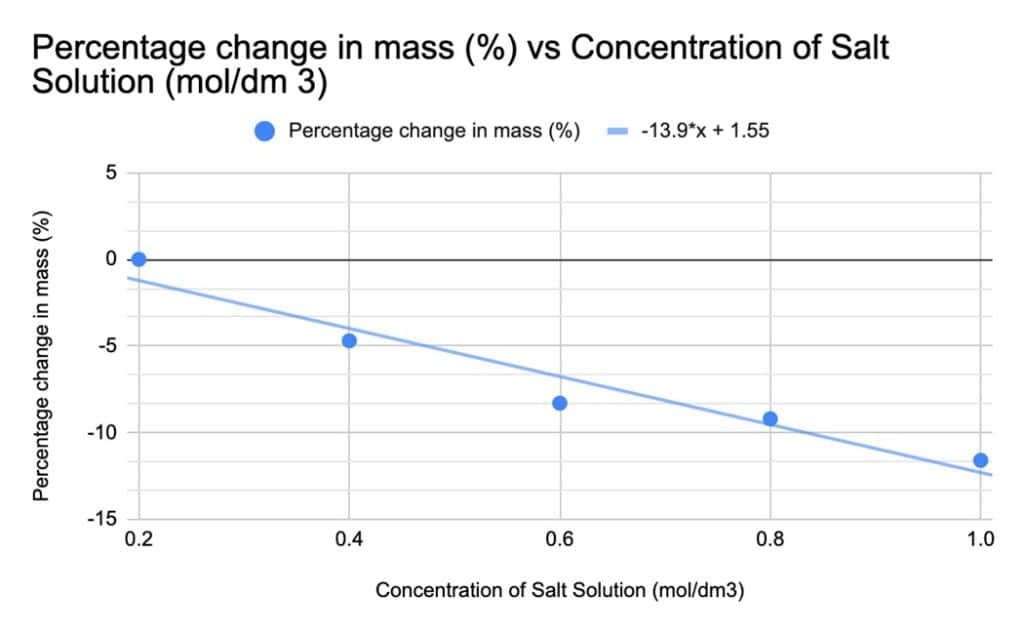
Results
| Concentration of Salt Solution (mol/dm 3) | Initial Mass of Cylinder Potato (g) | Final Mass of Cylinder Potato after (g) | Percentage change in mass (%) |
| 1 | 2.66 | 2.35 | -11.6 |
| 0.8 | 2.62 | 2.38 | -9.2 |
| 0.6 | 2.64 | 2.42 | -8.3 |
| 0.4 | 2.55 | 2.43 | -4.7 |
| 0.2 | 2.62 | 2.63 | +0.003 |
Qualitative Observations:
Although the colour of the potato cores did not change, potato cores placed in more concentrated salt solutions were a lot softer and flexible at the end of the experiment compared to the start as they were plasmolysed (Science Sauce, 2017). On the other hand, potato cores placed in solutions of lower salt concentrations were fuller ( they had more water molecules inside) and therefore were sturdier and harder than those placed in more concentrated salt solutions (Science Sauce, 2017).
Data analysis:
The potato cores exhibited a progressive decrease in mass as the salt solution concentration increased. This observation suggests that the salt solutions were hypertonic relative to the potato cores, implying a higher solute concentration in the salt solutions compared to the potato cells. However, at a salt solution concentration of 0.00 mol/dm3, the solution and potato cores were in an isotonic state, indicating an equal concentration of water molecules across the cell membrane. This implies that the potato cells were already fully turgid prior to immersion in water, rendering them incapable of absorbing any further water molecules. Consequently, water loss occurred at any salt solution concentration exceeding 0.00 mol/dm3. The equation of the line of best fit, y=-13.9x+1.55, suggests that the salt concentration would reach an isotonic state with the potato core at a molarity of 0.02 mol/dm3, aligning with the experimental findings.
Given the numeric and continuous nature of the independent variable, the concentration of salt solution in which the potato cores are immersed, a line graph (shown above) is the most suitable representation of the data. The graph reveals a linear and proportional relationship between the percentage change in mass of the potato cores (g) and the concentration of salt solution (mol/dm3). All data points align closely with the line of best fit, indicating the absence of anomalous results. The gradient of the line, y= -13.9x+1.55, implies that for every 0.25 mol/dm3 increase in salt solution concentration, the mass of the potato cores decreases by 3.127%.
The repeatability of the results obtained from this investigation is limited due to the collection of only one data point for each salt solution concentration instead of three. This single data point approach compromises the accuracy of the data as it prevents the calculation of a mean value from multiple measurements. Consequently, it becomes challenging to identify anomalous data points since there is no comparable data for reference. For instance, the percentage change in mass obtained at a salt solution concentration of 0.6 mol/dm3 was -8.3%, deviating from the -7.6% predicted by the line of best fit. This discrepancy represents a 34% difference compared to the line of best fit. However, due to the absence of additional data points, this deviation cannot be definitively classified as an anomaly. Moreover, the inclusion of additional data points might have altered the position of the line of best fit, potentially influencing the perceived accuracy of the mass change observed at a salt concentration of 0.6 mol/dm3.
The reproducibility of the experimental results is supported by their consistency with findings from a group with a similar method for finding the change in mass of potato cores at different concentrations of salt solutions. Despite both investigations utilising a single trial per concentration, rendering anomalous result detection challenging due to the lack of comparative data, both studies observed a consistent linear and proportional relationship between salt solution concentration and potato core mass change. As salt solution concentration increased, potato core mass decreased. The gradient obtained in the comparative study (-11.1) deviated from the gradient observed in this investigation (-13.9), representing a 20.14% difference. However, both gradients suggest a similar line trajectory. This gradient disparity could be attributed to variations in the initial water content of individual potato cores. Given that potato cores exhibit varying degrees of turgidity prior to experimentation, mass change results will differ unless multiple potato cores are tested at each salt solution concentration to calculate an average value.
Conclusion
The experimental data corroborates the principles of osmosis, as the potato cores exhibited a greater loss in mass when the surrounding solution possessed a higher concentration of water molecules (hypertonic) compared to the concentration within the potato cells.
Partially aligning with the initial hypothesis, the potato cores indeed experienced a mass decrease as the salt solution concentration increased, as depicted by the graph. However, the hypothesis regarding an increase in potato core mass upon submersion in distilled water was not supported. This discrepancy stems from the isotonic nature of distilled water with respect to the potato cores, which were already relatively turgid prior to immersion. Due to the comparable concentrations of water molecules between the potato cores and the surrounding solution, no net movement of water molecules, or osmosis, occurred across the cell membrane.
Evaluation
The experimental procedure introduced random errors in two key aspects related to the duration of potato core immersion in salt solutions. These errors stemmed from the time taken to initiate the timer after placing the potato cores in the respective solutions and the additional time the potato cores remained in the solutions after the timer elapsed. To mitigate these random errors, tweezers were employed to expedite the removal of potato cores from the salt solutions. Additionally, a single timer was used for all the cores, eliminating the potential for stacking errors. This approach ensured that each potato core experienced a similar duration of immersion, minimising the impact of random variations in timing on the overall results. Moreover, the maximum potential random error under this method is estimated at 10 seconds, translating to a possible 1% anomaly in the final outcome.
The experimental results showed high precision, tightly clustering around the theoretical predictions and aligning closely with the identified line of best fit. This level of accuracy is attributable to the effective minimization of systematic errors throughout the investigation. The balance used to measure the initial masses of the potato cores possessed an accuracy of 0.005 grams, resulting in an error margin of 0.19% given the target mass of approximately 2.62 grams. This negligible error would not have a discernible impact on the final outcomes. Additionally, the measuring cylinders employed exhibited an accuracy of 0.1 cubic centimetres. Considering that two measuring cylinders were utilised, the overall uncertainty in the results would be 0.2 cubic centimetres, with a range of 0.4 cubic centimetres. This translates to an error margin of 1.5%, which remains relatively low in the context of systematic errors. Moreover, as previously mentioned, the maximum possible random error incurred during the timing process would be 1%, aligning favourably with the two systematic errors encountered in this investigation.
The control variables were kept constant throughout. However, the masses of the potato cores could only be kept at a range of 0.03g between them as it was challenging to cut them to the nearest 0.005g. Nevertheless, this amounts to an error margin of 0.25% at most, which is more accurate than that of the other apparatus.
Extensions
To enhance the accuracy of the investigation, incorporating three trials for potato core mass measurements at each salt solution concentration would be beneficial. This would mitigate the impact of random errors and address the inherent variation in water molecule concentrations among individual potato cores prior to the experiment. Additionally, the surface area-to-volume ratio of each potato core, a factor not considered in this methodology, could influence the results. A larger surface area exposes a greater portion of the potato core to the surrounding salt solution, consequently affecting the rate of osmosis across the cell membrane. Therefore, controlling this variable is crucial for improving the precision of the findings. While directly controlling surface area-to-volume ratio is challenging, extending the immersion time of potato cores in the solutions, potentially for a day or more, can minimise the error associated with varying ratios. Prolonged immersion promotes a more uniform concentration of salt solution across the membrane, diminishing the significance of osmosis rate variations. However, if potato cores are left in the solutions for over a day, close monitoring of room temperature is essential, as temperature can accelerate the osmosis process.
Expanding the investigation to explore the effects of surface area on osmosis rates across potato cell membranes would be valuable. This could be achieved by maintaining a constant salt solution concentration and manipulating the surface area-to-volume ratio as the independent variable. Comprehending the implications of a larger surface area-to-volume ratio could eliminate the need for extended immersion times, as it would enable predictions regarding the impact of ratio changes on potato core mass.
Bibliography
BBC Bitesize, (n.d.). Transport across membranes, [Online], Available at: https://www.bbc.co.uk/bitesize/guides/zqdhjty/revision/6 [Accessed: 28th Nov. 2023]
Science Sauce, (2017). Osmosis in Potato Strips – Bio Lab, [Video], Available at: https://www.youtube.com/watch?v=jTDATlaBV-o [Accessed: 30th Nov. 2023]
Wikipedia, (2023). Osmosis, [Online], Available at: https://en.wikipedia.org/wiki/Osmosis [Accessed: 3rd Dec. 2023]

