Introduction
Every day substances are constantly being altered in some way. These altercations can be classified as physical or chemical changes. Physical changes are altercations in which the chemical composition of a substance remains constant. A common example is the change in the state of matter of dihydrogen monoxide (water – H2O). Its physical properties (state of matter, density, temperature) changed but the chemical composition still remains two hydrogen atoms and one oxygen atom.
Chemical changes are more complex in nature, instead involving changes in the substance’s chemical composition. This change in the chemical composition of a substance is the consequence of a chemical reaction. Simply put, a chemical reaction is defined as the restructuring of a substance’s molecular or ionic structure. One or more substances called the reactants form one or more new substances referred to as products. However, in the world of chemistry, there are many different chemical reactions. They are largely classified as part of one of five different types. This lab report focuses on the category of single-displacement reactions.
In a single-displacement reaction a more reactive element displaces another element from a compound often in the form of an aqueous solution. The substance that is displaced shows less tendency to undergo reaction; that is, it is less reactive. Thus, the force of attraction between the displaced element and the element in the compound is weaker than the attraction between the displacing element and the compound.
Single-displacement reactions only occur with metals and occasionally with halogens. For the purpose of this lab we will focus on reactions involving metals. A metal undergoing a single-displacement reaction displaces another metal from a compound. The metal that displaces the other metal does so due to its greater tendency to undergo reaction; it is more reactive. If metal A is more reactive than metal B, metal A will displace metal B from Compound BC. We can illustrate this as
A + BC → B + AC
The reactivity of a metal is a measure of its ability to compete in a single-displacement reaction. In an activity series, a sequence of metals is arranged based on this characteristic, from most reactive to least. The activity series is useful as an empirical tool for predicting the products of displacement reactions, and the reactivity of the reactants. Single-displacement reactions only occur under a certain set of conditions. For instance, it will only occur if metal or halogen A has
a higher reactivity (is higher in the activity series) than metal or halogen B. However, if A is less reactive (lower on the activity series) than B then no reaction will occur. This lab aims to determine the empirical reactivity of certain metals (including hydrogen) by conducting single-displacement reactions with aqueous ionic compounds. The results of this experiment and lab report will be compared to the actual activity series in an attempt to validate or corroborate the results.
Purpose
The purpose of this lab is to construct an activity series of metals using
empirical-qualitative data obtained from single-displacement chemical reactions between said metals and aqueous ionic compounds.
Hypothesis
If metal X is placed in an ionic aqueous solution, then the metal with higher reactivity (higher on the activity series) will displace the other metal, because its force attraction with the non-metal is stronger.
Equipment and Materials
- Seven small pieces of each of the following metals:
- Copper, Cu(s)
- Magnesium, Mg(s)
- Zinc, Zn(s)
- Sandpaper or Emery paper
- Dropper bottles containing dilute solutions (0.1 mol/L) of:
- Copper(II) sulfate, CuSO4(aq)
- Zinc sulfate, ZnSO4(aq)
- Magnesium sulfate, MgSO4(aq)
- Tin(II) sulfate, SnSO4(aq)
- Iron(II) sulfate, FeSO4(aq)
- Hydrochloric acid, HCl(aq)
- 24-well reaction plate
- Wash bottle with distilled water (Optional)
- Six test tubes (Optional)
- Test-tube rack
- Safety goggles
- Lab Coat (Optional); all other necessary personal protective equipment
Procedure
- Safety Goggles, a lab coat and necessary personal protective equipment were obtained before handling any materials
- Caution: Avoid unnecessary skin and clothing contact with clothing with the metals and/or solutions
- The materials and equipment outlined in “Suggested Materials” were obtained from the teacher.
- The oxide layer was removed from the metals by rubbing sandpaper or steel wool
- Obtained and placed the test-tube rack. Test tubes were placed within the rack.
- The 24-well Reaction plate was obtained.
- The copper was placed into each well in row one of the reaction plates.
- Five drops of copper(II) sulfate solution were placed into the first well of the reaction plate.
- Any observable qualitative changes were observed and recorded after one minute.
- Steps 7-8 were repeated with each solution and the distilled water until each copper containing well has been used in a reaction.
- Step 6-9 was repeated so that magnesium and zinc were reacted with each solution and distilled water.
- The waste beaker provided was utilized to effectively dispose of the solutions.
- The materials and equipment were cleaned and returned to their respective locations.
Observations
Table 1.1: Qualitative observations on the reactivity of metals with aqueous ionic compounds.
| Ionic Solutions (aq) | ||||||
| Metals | CuSO4 | ZnSO4 | MgSO4 | SnSO4 | FeSO4 | HCL |
| Copper | No reaction due to metals being the same element. | No Reaction | No Reaction | No Reaction | No Reaction | No Reaction |
| Zinc | Zinc strip begins to darken. Slight Bubbling indicates a gas. | No reaction due to metals being the same element. | No Reaction | Bubbling and a precipitate on the zinc were observed. | Color of iron sulfate changes and gray precipitate of iron is formed. | Bubbles vigorously as hydrogen gas is produced. |
| Magnesium | Bubbling and the formation of a black solid is observed. | Extreme Bubbling and the formation of a back solid is observed. | No reaction due to metals being the same element. | Vigorous bubbling was observed. | Slight bubbling. Change in color, turns black. | Significant Visible bubbles of, presumably hydrogen gas were observed. |
Results
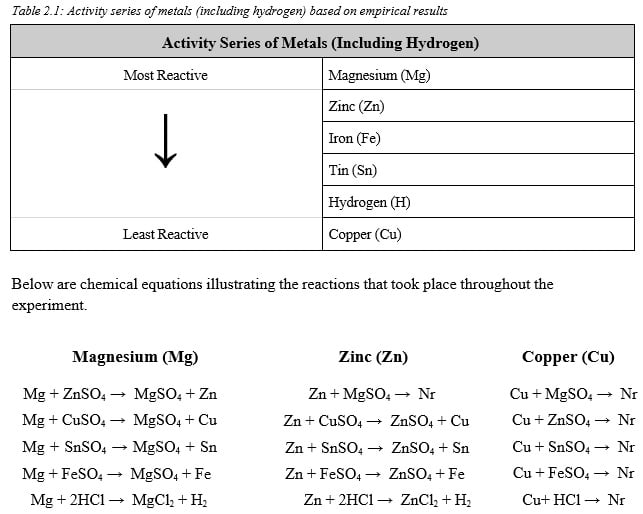
Discussion
After conducting an analysis of the qualitative empirical data derived from this experiment, it was concluded that magnesium was the most reactive metallic element. This is because the results show that magnesium was able to successfully displace every other metal, in the ionic aqueous solutions. On the other hand, solid copper was classified as the least reactive, due to its inability to displace any metals. It was evident through the experiment results that no chemical reactions involving solid copper took place. It was observed that zinc reacted with all
but two of the solutions. The first instance was with zinc sulfate, which could not have taken place since the metals are the same. The second instance was with Copper(II) sulfate. This put zinc behind Magnesium on the activity series since it reacted and successfully displaced metals one less time than Magnesium, which was successful all five times (disregarding Mg + MgSO4).
When determining the reactivity of Iron and Tin, the traditional method of counting the number of reactions could not be implemented since both elements successfully displaced two times. Instead, the qualitative observations were taken into consideration to help determine the reactivity of the two elements. The experimental data shows that Tin(II) sulfate reacts more vigorously with magnesium and zinc than did Iron(II) sulfate.
A more vigorous reaction is indicative of a larger difference in reactivity between Zinc/Magnesium and Tin relative to Iron and Zinc/Magnesium. Thus, Tin should be placed farther away from Zinc/Magnesium on the activity series. Based on this, Iron was listed as third place followed by Tin. Finally, the reaction between Zinc/Magnesium and hydrochloric acid was the strongest and most vigorous. Based on the same principles discussed above, hydrogen was placed after Tin. This is how the activity series listed above was derived.
Sources of Error
Throughout this experiment, several different sources of error could have altered the results or conclusions. The first source of error was the possibility of contamination. The materials utilized to conduct the experiment were old and thus would have accumulated a layer of oxygen, via a process known as oxidation. This layer of oxygen might not have been effectively removed from the elements due to their minuscule nature. The oxygen could have interfered with the results. Another source of contamination could have been water.
Water would have made its way into the reaction since it was used to rinse and clean the reaction wells. One could hypothesize that if the water was not removed from the reaction site, it could have interfered with the reaction. The last source of error was the fact that the experiment relied on qualitative data obtained from the human eye. Human observations are susceptible to observer bias. In other words, people could observe different results based on their expectations. Perhaps quantitative observations would be more accurate.
Conclusions
This lab aimed to construct an activity series of metals based on the variations in reactivity observed in the experiment. One can conclude that this report successfully attempted to create an accurate activity series, since the series created corresponds with the already established activity series of metals. The hypothesis of this experiment was also correct, in that elements with higher reactivities did displace those with lower reactivities.

