Enzymes are biological catalysts, responsible for directing the flow of chemical reactions that is the basis for life. By far the majority of enzymes are proteins, but some consist of RNA or a complex of protein and RNA. Enzymes are remarkably specific, acting on only one or a few types of molecules (called “substrates” of the enzyme) to give some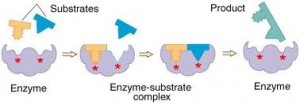 type of molecular product. In cellular metabolism, there are only a few major classes of enzymes that carry out the biochemical functions specific to their class. However, the enzymes within each class carry out these functions on very specific substrates that are specific to each enzyme. For example, there are many proteases, all catalyzing peptide bond breakage, but each protease has its own specialty (trypsin cleaves after K, R residues; chymotrypsin after F, W, Y; elastase after A, V, L; etc). It is the specificity and catalytic ability of enzymes that allows life to exist.
type of molecular product. In cellular metabolism, there are only a few major classes of enzymes that carry out the biochemical functions specific to their class. However, the enzymes within each class carry out these functions on very specific substrates that are specific to each enzyme. For example, there are many proteases, all catalyzing peptide bond breakage, but each protease has its own specialty (trypsin cleaves after K, R residues; chymotrypsin after F, W, Y; elastase after A, V, L; etc). It is the specificity and catalytic ability of enzymes that allows life to exist.
Enzymes, as catalysts, are not themselves changed by the reaction although they may change transiently during the reaction. They often cause huge rate enhancements (quadrillion-fold or more) but do not affect the basic driving force (thermodynamics) behind chemical transformations.
To understand how enzymes work, consider the course of a chemical reaction. Between the initial chemical reactant and the product is always an intermediate form called a “transition state.” In order for substrate (reactant) to become product (whether catalyzed or not), the substrate must pass through the transition state. If you consider the energy involved, the transition state is at the high point between reactant and product. For the reaction to occur, enough energy (the “activation energy”) must be input to raise the reactant to the transition state. Thus the rate of the reaction is controlled by the activation energy. Enzymes lower the activation energy by creating a favorable environment for the transition state. It is important to note, however, that the ratio of the substrate to product of the reaction at equilibrium (Keq) is not affected—only the rate.
Only a small part of an enzyme is directly involved in catalysis. This small part is called the “active site” of the enzyme. Often the active site is found in a cleft between two domains or subunits. This location allows the substrate to be surrounded and the reaction environment to be controlled by the enzyme. In the active site, multiple weak bonds are formed as substrate(s) bind, and the chemical and physical geometry of the site push the incoming molecule towards the transition state. An important point is that an enzyme has the highest affinity for the transition state of a reaction, not for substrates or products. Factors that contribute towards attaining the transition state include:
-bringing substrates together (in multi-substrate reactions)
- Orienting substrates in a favorable geometry
- Supplying proton acceptors/donors, electron donors/acceptors
- Excluding water
- Stressing the substrate physically or electronically
Enzymes work best under specific conditions of temperature and pH. Thus there is an optimal pH and temperature for each enzyme. Most enzymes are adapted to their natural conditions. For example, enzymes that work in the stomach have a low pH optimum. At extremes of pH, catalytic groups that are necessary for activity may be protonated (or unprotonated), leading to decreases in activity, or the enzyme may denature. With increasing temperature, the reaction rate increases up to the point at which the enzyme becomes unstable. Thus, for both temperature and pH, plotting activity gives a bell shaped curve. It is fascinating that some organisms live under extreme conditions of temperature, salt or pH, and enzymes have evolved in those organisms to cope with these conditions.
Case Study: Lysozyme
Lysozyme is an enzyme that is present in saliva, tears, egg white and other fluids. Lysozyme cleaves the bonds holding together polysaccharide chains that are present in some bacterial cell walls. Lysozyme is therefore thought to act as a natural antibiotic in these fluids. It is a relatively small globular protein, stabilized by disulfide bonds, and containing a mixture of secondary structures. A groove on the surface is the active site. The active site of the enzyme binds a polysaccharide, with six sugars interacting with the protein. Lysozyme catalyzes a hydrolytic reaction (ie. one that requires water) that severs the bond connecting two of the sugars.
X-ray structures of lysozyme with substrate analogs (chemicals that resemble the substrate but cannot undergo the reaction) show that lysozyme-substrate binding induces a distortion of one sugar, weakening the bond to be broken. Two acidic residues are precisely positioned adjacent to the bond where they receive and donate hydrogen ions. A series of electron movements results in the transient formation of an enzyme-sugar linkage at the same time as the sugars are separated. One product is then free to leave, and water is split to hydrolyze the enzyme linkage, releasing the remaining product and regenerating the active enzyme.
This reaction occurs millions of times faster than it does in the absence of catalyst and illustrates how enzymes create a favorable environment to speed up a reaction.
Enzyme Kinetics
“Kinetic” studies, the study of how the rate of a reaction is influenced by the concentration of substrate, can provide insight into an  enzyme’s mechanism and how inhibitors of enzymes act.
enzyme’s mechanism and how inhibitors of enzymes act.
At a fixed enzyme concentration, the rate of the reaction will increase as substrate concentration increases until a limit is approached. As the enzyme becomes saturated with substrate, further increases in substrate concentration do not cause increases in the rate of reaction. Graphically, this gives a rectangular hyperbola when initial velocity “v” is plotted against substrate concentration [S]. This curve is characteristic for many simple enzymes. In 1913, Leonor Michaelis and Maud Menten derived an equation to quantitatively describe this behaviour (known as the Michaelis-Menten equation):
v = Vmax[S]/(Km + [S])
where Vmax is the maximal velocity (velocity at infinite substrate concentration), and Km is a constant (called the Michaelis constant) that is equal to the substrate concentration required to give half the Vmax.
The Vmax of a reaction is directly dependent on the enzyme concentration (doubling the amount of enzyme will double the rate of product formation).
In part, the Km reflects the affinity of the enzyme for the substrate: the lower the Km, the higher the affinity. The Km is independent of enzyme concentration.
Because it is difficult to determine numerical values from a hyperbolic curve, data is often plotted using a reciprocal Lineweaver-Burk (double reciprocal) plot (1/v vs 1/[S]). This gives a straight line and allows determination of Vmax from the intercept on the 1/v axis and Km from the intercept on the 1/[S] axis.
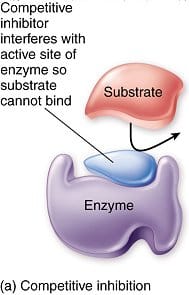
Inhibition of enzymes
Inhibiting enzymatic activity can be important and useful. For example, many antibiotics and medications are enzyme inhibitors. Inhibitors can be divided into 2 classes: irreversible (which form a covalent bond with enzyme) and reversible (which bind non-covalently). Penicillin is an example of an irreversible inhibitor, and permanently inhibits a bacterial enzyme that contributes to cell wall synthesis, thus inhibiting growth of the organism.
Many reversible inhibitors bind directly in the active site and block substrate binding – these are called “competitive inhibitors”. The kinetic effect is to increase Km. This type of inhibition can be overcome with high substrate concentration.