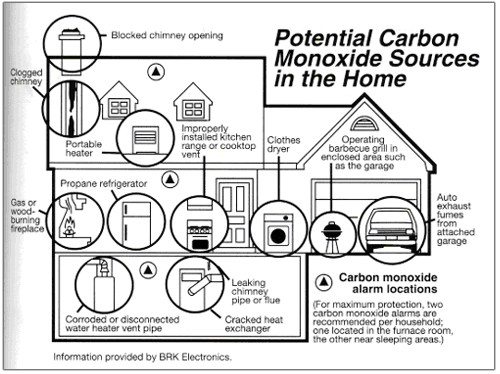
Summary
- Carbon Monoxide Detectors is a device that detects the presence of Carbon Monoxide (CO)
- Excess Carbon Monoxide(CO) = Carbon Monoxide Poisoning
- Silent Killer
- Incomplete Combustion
History of Dalton’s Law
- John Dalton (6 September 1766 – 27 July 1844) was an English Chemist, Meteorologist and physicist
- He investigated and published the atomic theory which states
- Elements are made of tiny particles called atoms.
- The atoms of a given element are different from those of any other element; the atoms of different elements can be distinguished from one another by their respective relative atomic weights.
- All atoms of a given element are identical.
- Atoms of one element can combine with atoms of other elements to form chemical compounds a given compound always has the same relative numbers of types of atoms.
- Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process; a chemical reaction simply changes the way atoms are grouped together.
Daltons Law
- The pressure of a mixture of non-reacting gases is the sum of the partial pressures of the individual gases in the mixture
- · PTotal = P1 + P2 + P3
- The partial pressure is the pressure that one gas exerts on the sides of the container
- Mole Fraction: PA = Ptotal x na
Daltons Law and CO
- Partial pressure of CO is that of which would be Carbon alone and Oxygen alone
- Pressure of Carbon and Oxygen combined = Total Pressure
- Enclosed space causes increase in pressure
- Dangerous if CO is not ventilated properly and confined in a small area
How do CO Detectors Work?
- 3 Different Types
- Colorimetric detectors
- Metal-oxide detectors
- Electrolytic detector
How is CO Produced?
- Carbon monoxide is produced from the partial oxidation of carbon-containing compounds
- Operating Stoves and Internal Combustion engine in enclosed space
- Using Air freshener after painting walls
What Does CO Do To Your Body?
- Combines with haemoglobin.
- Carbon monoxide is 200 times better at combining with haemoglobin than oxygen
- At a level of 0.1%, carbon monoxide will kill us quickly
- Carbon Monoxide Detectors & Economy
- High demand = High price
- Required in many places
- Large profit for many businesses
- Carbon Monoxide Detectors & Society
- Many individuals educated about risk
- Apart of curriculum for fire fighters
- Many take part in learning how to install and repair detectors
Carbon Monoxide Detectors & Environment
- Many detectors now made environmental friendly
- They can cause risk to environment if not functioning proper
- Must be disposed of carefully if there is CO still trapped inside