After collecting the following data and observations, the unknown liquid, #19UL11, was determined to be 2-Butanone. 2-Butanone is a ketone with a boiling point of 80 degrees Celsius, and a molecular weight of 72.11 g/mol. This data was confirmed by the boiling point determined in lab which was 68 degrees celsius, and from the GCMS data (refer to Table 2) .
Table 2. Mass Spectral Data
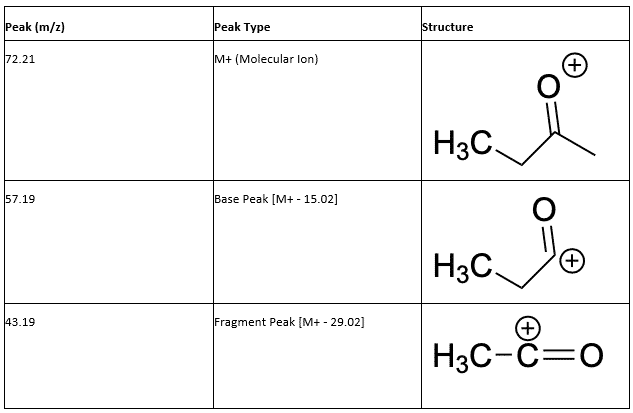
Within the mass spectral data, the M+ peak was labeled as 72.21 m/z, which corresponds to the parent compound of the unknown liquid. In the IR spectrum (Table 1), there is a peak at 1712.02 cm–1 which indicates the presence of a carbonyl in the structure of the unknown. The two other peaks, one being 2979.39 cm–1, and the other at 2939.99 cm–1 shows the presence of sp3 hybridized C-H bonds, which correspond to the methyl groups.
In Table 3, the 1H NMR data shows a triplet with an integration of 1 at 1.05 ppm. This corresponds to the carbon that has two neighbors and is attached to the carbonyl carbon. There is another peak at 2.1 ppm which is a singlet and has an integration of 3 as well. This peak corresponds to the methyl group connected to the carbonyl carbon, making it a ketone. The last peak is a multiplet at 2.5 ppm with an integration of 6. This corresponds to the two methyl groups on the structure.
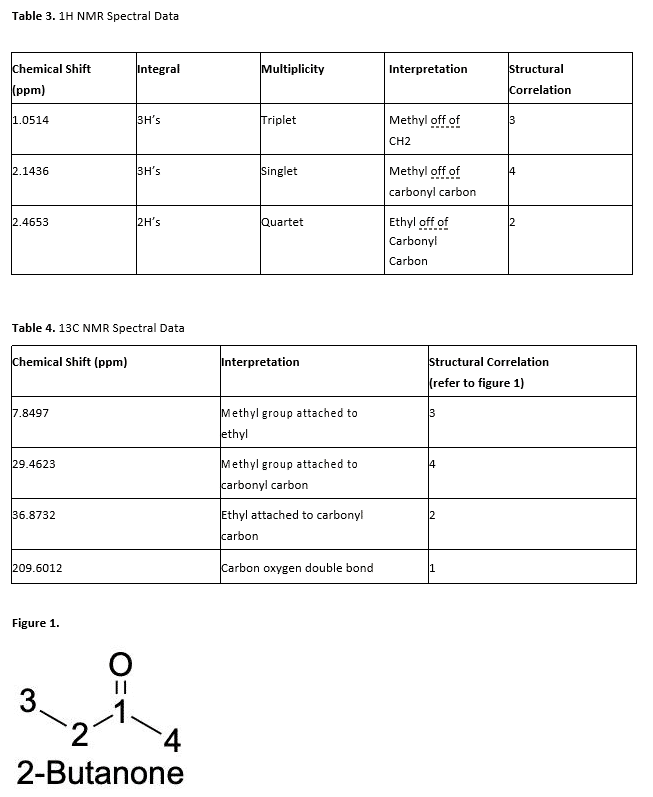
The 14C NMR data (table 4) represents the carbons that are present in the molecule. All the carbons shown on this spectrum indicate odd numbers of attached H’s, and have peaks at 7.8 ppm, 29.4 ppm, 36.9 ppm, and 209.6 ppm. This information allows for the conclusion that there are no CH2 bonds in the structure, only sp2hybridized C-H bonds and methyl groups.
In order to determine which classification tests to run, the information listed above aided in choosing which tests would give a positive result for aldehydes. Based on this information, the following tests will be run: 2,4-dinitrophenylhydrazine, Potassium Permanganate (also known as Baeyer’s Test) and the Iodoform Test. After deciding which tests to run, controls needed to be chosen based on what would provide a positive versus a negative result.
2,4-dinitrophenylhydrazine classification tests positive for ketones and aldehydes, and negative for alcohols. Therefore, the controls will be 2-butanone (positive) and tert-amyl alcohol (negative). Potassium Permanganate, also referred to as Baeyer’s Test is said to show positive results for alkenes or alkynes and negative results for alkanes. Thus, the controls for the last classification tests are benzaldehyde (positive) and 1-hexanol (negative).
Lastly, the Iodoform test shows positive results for ketones, and negative results for aldehydes. Based on the previously proposed classification tests, this test will be the determining factor in confirming that the unknown is a ketone. The controls for this test will be cyclohexane (negative) and 2-butanone (positive).
Using the spectral data collected, there are multiple points which proves that the unknown is indicative of an aldehyde. Some of these points include a strong broad peak at 2939.99 cm–1 on the IR data, and a peak on the 1H NMR at 2.1 ppm that is a singlet with an integration of 3. This peak is indicative of a methyl group next to a carbonyl which is a part of the unknown aldehyde. The following two tests show positive results for aldehydes, and the previous data supports the proposal for these tests.
2,4-dinitrophenylhydrazine, also known as Brady’s test, is used to test for the carbon-oxygen double bond that is present in ketones and aldehydes. In order to perform this test, drops of the unknown (2-methylpropanal), and the two controls, 2-butanone (positive result) and tert-amylalcohol (negative result), will be added to the Brady’s reagent.
This reagent is composed of a solution of 2,4-dinitrophenylhydrazine in methanol and sulfuric acid. When the drops are added to the reagent, a bright yellow/orange precipitate will appear yielding a positive result. This precipitate confirms the presence of the carbon-oxygen double bond that is present in aldehydes and ketones. The reaction itself is a condensation reaction because two of the reagents combine with one another after losing a molecule of water.
The mechanism of this reaction is considered a nucleophilic addition-elimination reaction. In the first steps of the mechanism, the Brady’s reagent adds across the carbonyl’s double bond resulting in a tetrahedral intermediate. The intermediate then loses a molecule of water to create the final product. The R groups on the scheme below indicate either a hydrogen or hydrocarbon group, which ensures that the molecule is a ketone or an aldehyde.

Based on the data presented in Table 5, both the unknown solid and one of the controls yielded a positive result. This confirms that the unknown is either a ketone or an aldehyde. These results provide evidence for the next test for the presence of a carbon-oxygen double bond, which can be accomplished in the Potassium Permanganate test.
Table 5:2,4-Dinitrophenylhydrazine
| Controls | Observations | Results |
| Unknown | Formed a bright orange | positive |
| precipitate | ||
| Tert-amyl alcohol | Formed a transparent orange | negative |
| liquid | ||
| 2-butanone | Formed bright orange | positive |
| precipitate | ||
Potassium Permanganate test, also known as Baeyer’s Test, is based on the ability of potassium permanganate to oxidize carbon-carbon double and triple bonds, thus making it an oxidation reaction. This occurs because the alkene is replaced by a negative OH group, and the charge changed from positive one to positive 2, meaning there is a loss of one electron in the mechanism. This can be translated to the molecule being oxidized to a 1,2 diol. Within the experimental portion of the test, the permanganate is reduced, and a colored precipitate is formed (most likely brown).
To determine whether the test is positive or negative, the color must disappear. At first, this test was chosen to provide a negative result for the unknown aldehyde, but after researching results of this test, it was found that aldehydes, or other functional groups that contain a carbonyl group will also cause decolorization. Therefore, it is to be expected that the unknown will indeed provide a positive result during the test. The controls being used in this test are benzaldehyde (yielding a positive result) and 1-hexanol (yielding a negative result). The spectral data providing this evidence is from the 13 C NMR which displays peaks at only odd ppm.
This is indicative of single CH bonds, and methyl groups, which means that there can be no carbon-carbon double bonds present. The reason that the unknown liquid will yield a positive result even though there are no alkenes or alkynes present is due to the fact that the tollens reagent will oxidize the aldehyde. Therefore, by using this test, it will provide positive results for a carbonyl group, but not an alkene or alkyne.
The results which accumulated after completing the Potassium Permanganate classification test provide evidence of a carbonyl group (carbon-oxygen double bond). Referring to Table 6, both the unknown liquid and the control, 1-hexanol, provided negative results, meaning that there are no carbon-carbon double bonds within the unknown. This verifies that there is only a carbon-oxygen double bond. The last step to determining the unknown was to differentiate between a ketone and an aldehyde, which the Iodoform test will accomplish.
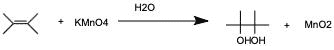
Table 6: Potassium Permanganate
| Controls | Observations | Results |
| Unknown Liquid | Deep purple color | Negative |
| 1-Hexanol | Deep purple color | Negative |
| Benzaldehyde | Brown liquid w/ a brown solid | Positive |
The main purpose of the Iodoform test is to be able to distinguish between a ketone and an aldehyde. The component of the test that uses iodine, will allow for a positive or negative result to be observed. This is because ketones that contain a methyl group that is directly adjacent to the carbonyl will react with the iodine. If positive result, the reaction tube will contain an opaque yellow solid, also known as Iodoform.
Another distinguishing characteristic of this product is its potent smell of antiseptic. The chemistry behind this test begins with the substitution of all the hydrogen in the methyl groups attached to the structure. This leaves hydroxide anions which are essential to the reaction in terms of a curved arrow mechanism. In the next step, the molecule is broken down to produce iodoform, which will be a yellow product. If this does not occur, then the molecule in the starting material was not a ketone.
Using the unknown liquid as the main part of the experiment, the two other controls, (refer to table 7) were used to create a reference point. Both the unknown liquid and the
2-Butanone yielded a pastel opaque yellow liquid. This proved that both starting materials were ketones. In other words, this is also conformation of the methyl group having three hydrogens and not just one, which would be indicative of an aldehyde not a ketone.
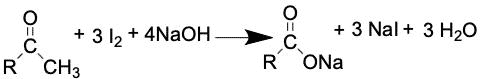
Table 7: Iodoform Test
| Controls | Observations | Results |
| Unknown Liquid | Pastel, opaque yellow color | Positive |
| Cyclohexane | Milky yellow, but somewhat | Negative |
| translucent | ||
| 2-Butanone | Pastel, opaque yellow color | Positive |
When forming the derivative, the contents from the 2,4-dinitrophenylhydrazine test was reused. When put through the Hirsch funnel, the product turned an orange color. When taken out of the Hirsch funnel and pressed between two pieces of filter paper, some of the product stuck to both sides of the filter paper resulting in the loss of some product.
After being dried, the melting point was taken and was established as 199.9 degrees celsius. The actual melting point of the derivative had a range of 118-119 degrees celsius. This confirms the purity of the substance, therefore allowing the conclusion that the unknown liquid #19UL11, is 2-Butanone.
Spectroscopy Data
Table 1. IR Spectral Data
| Peak (cm-1) | Description | Bond | Functional Group |
| 1712.02 | Strong, sharp | C = O | Carbonyl |
| 2939.99 | Weak, broad | C – H | Alkyl (sp3) |
| 3979.39 | Weak, sharp | C – H | Alkyl (sp3) |
