- After reviewing the Bohr-Rutherford diagram you should know that atoms wish to “fill” electron orbits.
- Atoms tend to combine in a way that allows the resultant compound to be neutral but have only full electron orbits on the atoms.
Lets do an example:
Note: I draw the electrons with holes in noticeable places. In reality the electrons space themselves evenly in an orbit 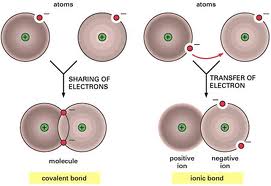 to maximize distance from other electrons (repulsion)
to maximize distance from other electrons (repulsion)
- Pictured here are two atoms. Notice that sodium has a single electron in its outer orbit while chlorine is missing a single electron from its outer orbit
- By “giving” a single electron to chlorine, sodium can have its orbits full (or empty) and chlorine can have its orbits full.
- This transfer of electrons causes the atoms to develop a charge (the metal is losing electrons & the non-metal is gaining electrons)
- We know that opposite charges attract which is what causes these two atoms to stick together forming a compound
- This compound is called sodium chloride (NaCl) also known as table salt.
- Metals always LOSE electrons, they always gain a POSITIVE charge, they can ONLY bond with non-metals
- Non-metals tend to GAIN electrons, they tend to have a NEGATIVE charge
- The “hooks” that we represented earlier are actually the extra electrons (or electron openings) that each atom possesses
- Atoms rarely have more then four “hooks”. This is because when an atom has four electrons in its outer orbit it can either gain four or lose four to have only full orbits.
- If the atom has more then four electrons in its outer orbit it is more likely to gain electrons to fill the orbit
- The hooks, when joined show the transfer of electrons from one atom to another
- The transfer of electrons results in the compound having a charge of zero while the atoms that make it up are charged
- If you compare your “combining capacity” tables with your Bohr-Rutherford diagrams you’ll notice that they relate directly to the number of electrons (or electron spaces) on the atoms outer orbit
- You’ll notice that all elements in the same COLUMN have the same number of electrons in their outer orbit
- You’ll also notice that all elements in the same ROW have the same number of electron orbits
- If the atom has more then four electrons in its outer orbit it is more likely to gain electrons to fill the orbit
- If the atom has fewer then four electrons in its outer orbit it more likely to lose those electrons to end up with only full orbits
- Thus, most atoms will not have more then four “hooks” or bonds that they can form with other atoms.
- Look carefully at your combining capacity charts and your Bohr-Rutherford diagrams and see if you can determine the pattern present in the first 20 elements relating to the number of bonds they can form (hooks)
- All Your Base Are Belong To Us
- Column one (I)
- = one electron in outer orbit
- = one electron must be moved (lost) to “fill” orbits
- = combining capacity of one
- Column two (II)
- = two electrons in outer orbit
- = two electrons must be moved (lost) to “fill” orbits
- = combining capacity of two
- Column thirteen (IIIA)
- = three electrons in outer orbit
- = three electrons must be moved (lost) to “fill” orbits
- = combining capacity of three
- Column fourteen (IVA)
- = four electrons in outer orbit
- = four electrons must be moved (lost/gained) to “fill” orbits
- = combining capacity of four
- Column fifteen (VA)
- = five electrons in outer orbit
- = three electrons must be moved (gained) to “fill” orbits
- = combining capacity of three
- Column sixteen (VIA)
- = six electrons in outer orbit
- = two electrons must be moved (gained) to “fill” orbits
- = combining capacity of two
- Column seventeen (VIIA)
- = seven electrons in outer orbit
- = one electron must be moved (gained) to “fill” orbits
- = combining capacity of one
- Column eighteen (VIIIA)
- = eight electrons in outer orbit (two for helium)
- = all orbits are full of electrons in natural state
- = combining capacity of zero
- Columns three – twelve (IIIA – IIB)
- = called the “transition” metals
- = each atom can have different numbers of electrons in its
outer orbit. (Multi-Valent) - = since they are metals they will still LOSE electrons
gaining a POSITIVE charge
Multi-valence atoms
- The transition metals (columns 3 – 12) can often have different combining capacities
- Iron for example can have a combining capacity of either 2 OR 3
(That means it can have either 2 OR 3 electrons in its outer electron shell) - When representing these atoms as words we write the combining capacity as a roman numeral following the metal
Iron (II) oxide OR Iron (III) phosphide
Types of Bonding
- When atoms bond together there are two ways in which this can occur
- Ionic Bonding
- Covalent (molecular) bonding
Ionic Bonding
- Ionic bonding involves both a metal and a non-metal
- The metal will give up its electrons to the
non-metal. (thus they both become IONS) - The metal becomes a positive ion (it lost electrons)
- The non-metal becomes a negative ion
(it gained electrons) - Because one atom is negative and the other is positive now, the atoms are attracted to each other. (Electrostatics!)
- Ionic compounds dissolve in water easily
- The ions separate and become surrounded by water. This separation allows electricity to pass easily through the solution.
- Later we will see that molecular bonded substances will NOT conduct electricity.
Covalent Bonding
- In ionic bonding, electrons were taken from one atom by another creating ions which then held together due to electromagnetic forces (opposite charges)
- Covalent (molecular) bonding is a process by which two atoms SHARE electrons. (Hence the name CO-VALENT)
- A covalent bond is when a pair of electrons is shared between two non-metal atoms to create a stable molecule and hold the atoms together
- Shared electron pair
- NON-metals only
- Stable molecule
- Holds atoms together
- Electrons orbit BOTH atoms
