Table 1 – All Attempted Trials Between The Acid “Unknown B”, And The Base, (NaOh).
| Trial | Initial Burette Volume Reading (mL) | Final Burette Volume Reading (mL) | Volume Of Base (Titrant) Added (mL) | Success Or Fail |
| 1 | 0.00mL | 8.20mL | 8.20mL | Failed |
| 2 | 8.20mL | 16.15mL | 7.95mL | Success |
| 3 | 16.2mL | 24.0mL | 7.80mL | Success |
| 4 | 32.0mL | 39.9mL | 7.90mL | Success |
| 5 | 33.3mL | 39.7mL | 6.40 mL | Failed |
| *Trial 5 was an outlier: potential contamination or measurement error lead to this result. | ||||
Table 2: Successful Titration Trials Between Acid “Unknown B”, And Base, (NaOh), Where Endpoint Was Achieved
| Trial | 2 | 3 | 4 | Average |
| Initial Burette Volume Reading (mL) | 8.20mL | 16.2mL | 32.0mL | – |
| Final Burette Volume Reading (mL) | 16.15mL | 24.0mL | 39.9mL | – |
| Volume Of Base (Titrant) Added (mL) | 7.95mL | 7.80mL | 7.90mL | 7.88mL |
Goal: Find the concentration of acid “Unknown B” if 10mL of acid was neutralized completely with a base solution of sodium hydroxide that has a concentration of 0.2 mol/L.
Givens:
Concentration Of Base NaOH = 0.20 mol/L Average Volume of Base NaOH = 7.88mL Volume of Acid “Unknown B” = 10.0 mL
Calculating/Solving For The Concentration Of Acid “Unknown B”:
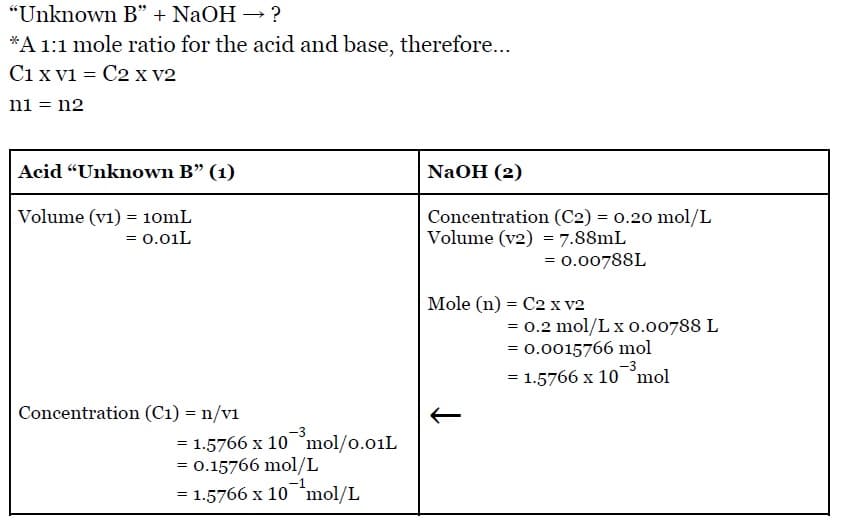
Conclusion:
Therefore, the concentration of the unknown acid “Unknown B” is 1.5766 x 10mol/L.
