- Isotopes have the same number of protons, but different numbers of neutrons.
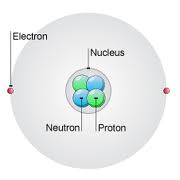
- This means that while the atomic number of an element will NEVER change…
The MASS of the atom can change quite a bit depending on how many neutrons there are - The number of protons in an atom is also called the “atomic number”. This number is ALWAYS listed for you in a periodic table of elements.
- The mass number listed in your periodic table of elements is for the MOST COMMON ISOTOPE of that element, other isotopes do exist but this information would have to be provided to you
- The number of Neutrons can be calculated using the following equation:
# of Neutrons = Mass Number – Atomic Number - Number of Electrons
> If an atom is neutral overall (positive and negative charges are canceled) then:
# of electrons = # of protons.
If the atom has a charge then that charge determines the number of electrons added or lost from the atom. (the same way it will in the electricity unit) - When an atom has an unbalanced number of protons and electrons it is given the name “ion”
- Ionic atoms will be indicated by their exact charge in the upper right corner of their symbol
- Atoms with a negative charge have more electrons than protons
- Atoms with a positive charge have fewer electrons than protons
- The equation for # of electrons can be arranged three ways:
- #protons – #electrons = total charge
- #protons – total charge = #electrons
- #protons = #electrons + total charge
If anybody keeps telling me that protons move around my head might explode. You now know why protons don’t move. (protons determine elements, if they moved the atoms would keep changing elements)
PROTONS NEVER EVER MOVE!!!!!!!
