The shape of a molecule determines many of its properties; For example: C4H10 is the formula for 2 separate molecules
n-butane and isobutene
Melting Point -138°C -159°C
Boiling Point -0.5°C -12°C
Enzymes—shape compatible with those molecules whose reactions they promote
i.e. the enzyme sucrase breaks down the sugar molecule sucrose
The shape of a molecule is described in terms of;
bond length/ bond angle
When atoms form molecules, their shapes will be determined by the repulsion of electrons about each atom. Since all things tend to lowest energy, the electrons (in bonding pairs) will tend to get as far away from each other as possible (or, in other words, have the bonds separated by the largest possible bond angle) in order to reduce the amount of repulsion and lower the energy. This is called the VSEPR theory.
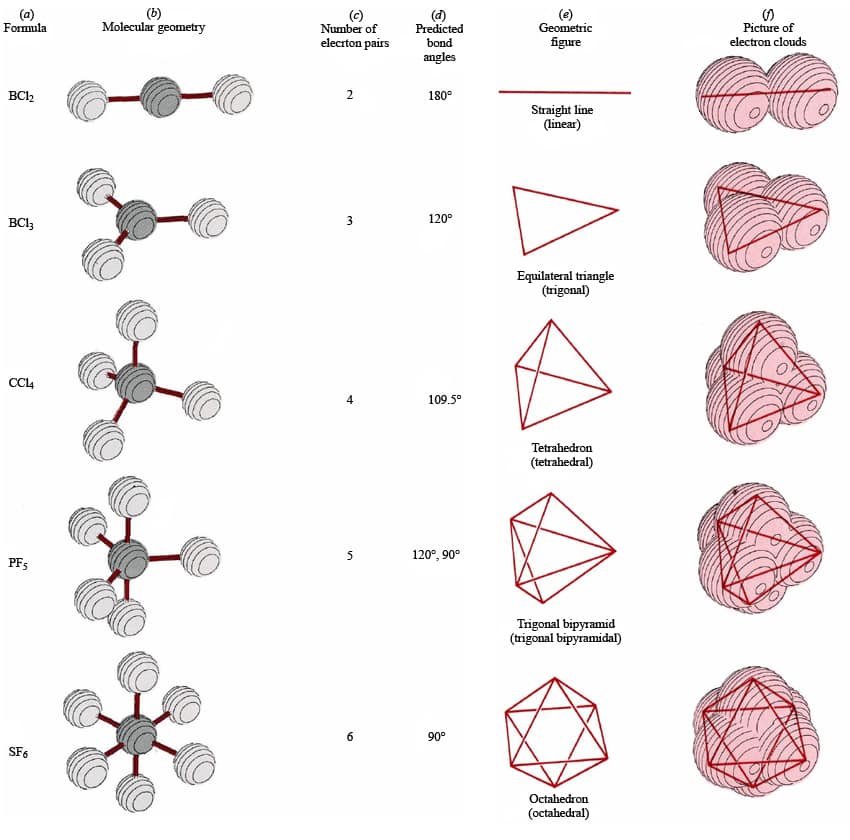
Bond angle is determined by the arrangement of electron pairs around the central atom and this in turn is determined by the number of electron pairs.
Predicting Molecular Shape Using VSEPR
- Draw a preliminary Lewis model where;
- Bonding pairs represented by a bar/line
- Lone pairs/Non-bonding electrons represented by dots
- Determine total number of electron groups around central atom (A)
- Count each bond–single, double, or triple–as one VSEPR pair (X)
- Count each lone (nonbonding) pair as one VSEPR pair (E)
Note: Lone pairs are counted because they repel neighbouring pairs and thus influence the positions of bond pairs.
- Determine the geometric arrangement that will accommodate this total number of electron groups.
- Arrange VSEPR pairs around the central atom so that they are as far apart as possible so as to minimize repulsions between them
| VSEPR Pairs | Geometric Shape |
| 2 | Linear |
| 3 | Trigonal Planar |
| 4 | Tetrahedral |
| 5 | Trigonal Bipyramidal |
| 6 | Octahedral |
- Draw molecular shape from positions of bonding pairs and lone pairs (i.e. VSEPR and bond angles).