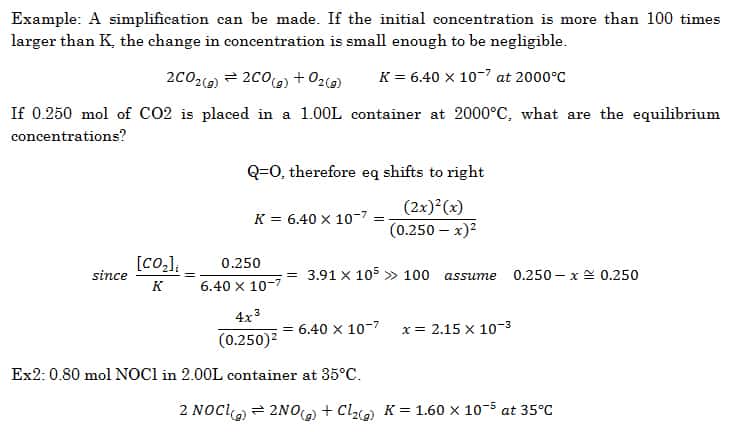
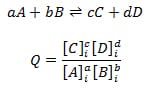The reaction quotient is an equation that allows us to determine whether a system is at equilibrium or not.
The equation is the same as the equilibrium law except the concentrations are initial concentrations instead of equilibrium concentrations.
- If Q = K the system is at equilibrium
- If Q > K, the system will shift to the left because the product concentrations are higher than their equilibrium amounts
- If Q < K, the system will shift to the right because the product concentrations are lower than their equilibrium amounts