Seven Characteristics of Life
- Display Order
- Arranged in a highly ordered manner
- Cell: fundamental unit of life
- Harness & Utilize Energy
- Acquire energy from the environment and use it to maintain state
- Reproduce
- Have the ability to make more of their own kind
- Respond to Stimuli
- Can make adjustments to their structure, function and behavior in response to changes to external environment
- Exhibit Homeostasis
- Regulate internal environment so that conditions stay relatively constant
- Growth & Development
- Increase their size by increasing the size/number of cells
- Evolve
- Populations change over generations to become better adapted to the environment
The Fundamental Unit of Life
- Cell Theory
- All organisms are composed of one or more cells
- The cell is the smallest unit that has the properties of life
- Cells arise only from the growth and division of preexisting cells
LUCA
- Last Universal Common Ancestor
- Evidence:
- Lipid membranes
- Genetic system based on DNA
- Reproduction
- ETC – ATP & Glucose
- DNA to RNA to Protein transfer of information
- Common system of protein assembly: ribosomes, mRNA, tRNA
Earliest Life
- Stromatolites dated to 3.5 billion years ago represent the earliest fossil evidence of life
- Layered rock that is formed when microorganisms bind articles of sediment together, forming thin sheets
- Formed by cyanobacteria – modern – posses a sophisticated metabolism
- Panspermia is the hypothesis that very simple forms of life are present in space and seeded the earth soon after it cooled
Stages of Prebiotic Evolution
- So what do you need?
- Abiotic synthesis
- Heritable Information
- Formation of Cells
- Assembly of complex organic molecules from simple molecules, including protein, RNA or both
- Aggregation of complex organic molecules inside membrane-bound protobionts
Geophysical Stage
- Conditions On Young (Primordial) Earth
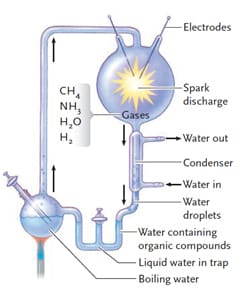
- Chemicals: H2O, H2, CH4, NH3, H2S
- Energy Sources: ultraviolet light, lightening
- Reducing atmosphere
- Allow for building up of highly reduced compounds (electrons)
- Miller-Urey experiment demonstrated that abiotic synthesis of biologically important molecules such as amino acids, sugar, nucleotide bases, lactic, acetic, formic acid, is possible
- 1953: Miller and Urey tested this theory and produced organic molecules from inorganic ingredients
- Life may have evolved in deep sea vents, atmosphere or surface of earth
Chemical Stage
- Abiotic Synthesis: organic molecules from inorganic molecules
- No abiotic synthesis today – oxidizing environment
- Reactants
- Water vapor
- Ammonia
- Methane gas
- Hydrogen gas
- Conditions
- Results (after 1 week)
- Aldehydes, carboxylic acids formed
- Glycine and alanine formed
- Amino acids
- Sugars
- Purines & pyrimidines
Chirality
- Chiral molecule: not superimposable on its mirror image (come in two forms)

- Two enantiomers (optical isomers)
- Handedness
- Same chemical and physical properties
- Different biological properties
- Thalidomide
- Used as a “morning sickness” drug in 1960s
- Antiemetic & readily convert to the other chiral form (teratogen causing birth defects)
- Approved in 20 European countries not in NA
- Side effect: deformed children
- Chirality Problem
- Miller-Urey experiment = racemic (50% of both chiral forms mixture)
- Biology is homochiral (only one form, no mixture)
- L amino acids, D sugars
Origin of Homochirality
- Homochirality
- Essential to the evolution of life
- Specificity is required = one chiral form
- Random chance
- Extraterrestrial origin
- Murchison meteorite
- Contains 7 amino acids
- 9% more L isomer (bias led to life)
- Murchison meteorite
Biological Stage
- Development of DNA, RNA and protein triad
- Synthesis of polymers
- Monomers not polymers – first cells (Miller-Urey experiment)
The Origins of Information & Metabolism
- All organisms contain deoxyribonucleic acid (DNA)
- DNA is copied onto ribonucleic acid (RNA)
- RNA directs the production of protein molecules
- Enzymes catalyze all reactions
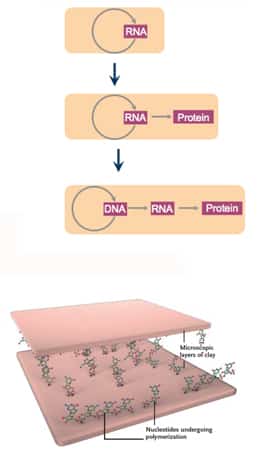
Enter RNA World
- RNA: information, structure, catalysis
- Speed up rate of reaction
- How can RNA catalyze?
- RNA can fold
- Complementary base pairing
- Ribosome
- Ancient organelle – required for all cells
- 2/3 RNA, 1/3 protein
- RNA can fold
- Ribozymes
- RNA molecule that has catalytic properties
- Self-splicing introns – catalyze own excision
- Can catalyze reactions on the precursor RNA molecules that lead to their own synthesis, as well as on unrelated RNA molecules
- Ribosome aminotroinferase activity
- Can fold into very specific shapes and are single-stranded
- Function depends on folding
- RNA was the first molecule from which both DNA and proteins developed
- Ribosome aminotransferase activity
- RNA molecule that has catalytic properties
Evolution of Information Transfer
- RNA
- Information, catalysis & structure
- Proteins
- Structure & catalysts
- Diversity (20 amino acids)
- DNA
- DNA is more stable than RNA
- Deoxyribose more stable then ribose
- Base uracil found in RNA is not found in DNA; replaced by thymine – common mutation in DNA is the conversion of cytosine into uracil – by utilizing thymine in DNA, any uracil is easily recognized as a damaged cytosine and can be repaired
- DNA is double-stranded – complementary strand can be used to repair the damaged strand
- DNA is more stable than RNA
The First Cells
- Monomers -> polymers: abiotic synthesis of cell molecules
- Hypothesis
- Clay particles creating a surface upon which polymerization reactions could occur
- Accelerated spontaneous reaction by clay particles (montmorillionite)
- High surface area & charged surface
- Charged components of the molecules are attracted to the charged surface of the clay
- Facilitate formation of SHORT nucleotide/amino acid chain (still function and have a selective advantage)
- Clay particles creating a surface upon which polymerization reactions could occur
- Flourescent Dye Evidence
- Purpose: how long fluorescent dye is retained in an abiotically synthesized vesicle
- Separates biological environment from outside environment
- Retain macromolecules inside so their concentrations are higher inside
- Protobionts: The First Cells
- Abiotically produced organic molecules that are surrounded by a membrane or membrane-like structure
- Formation allowed for an internal environment to develop that was different than external environment

