Objective:
In this lab, we will perform the catalyzed decomposition of hydrogen peroxide under various conditions. We will record the trials using a gas pressure sensor in a lab quest and analyze graphs of the data. From this analysis, we will determine the rate constant, the activation energy and the rate law for the reaction.
Pre- Lab Questions:
1. The rates obtained in this lab will be obtained using a pressure sensor, therefore your unit for rate will not be the typical M/sec. Your rates will be in a pressure unit/sec. Using the Ideal Gas Law, convert a rate of 0.56 kPa/sec into molarity per second.
0.56 kPa x 1 atm x 1 Molarity = 2.26 x 10^-4 M/sec
1 sec 101.3 kPa (0.0821)(298)
PV = nRT
P/RT = n/V
n/V = M
M = P/RT
2. The hydrogen peroxide solution that you are using in this experiment is labeled as a 3% solution, mass/volume (3 g H2O2 per 100 mL of water). However, in order to complete the calculations, the concentration must be in molarity.
Calculate the molarity of ta 3% mass/volume H2O2 solution (Part I, II, IV) and a 1.5% mass/volume H2O2 solution (Part III) and record these values in the table below.
| Part | Volume H2O2 (mL) | [H2O2] before mixing | Volume KI (mL) | [KI] before mixing |
| I | 4 | 0.882 M | 1 | 0.5 M |
| II | 4 | 0.882 M | 1 | 0.25 M |
| III | 4 | 0.441 M | 1 | 0.5 M |
| IV | 4 | 0.882 M | 1 | 0.5 M |
3g H2O2 x 1 mol H2O2 x 1000 mL = 0.882 M
100 mL H2O 34g H2O2 1 L
1.5g H2O2 x 1 mol H2O2 x 1000 mL = 0.441 M
100 mL H2O 34g H2O2 1 L
MSDS:
H2O2: Clear odorless solution. Keep away from heat. Keep away from face, skin, and eyes.
KI: Clear odorless solution. Keep away from skin, face and eyes. Keep this solution well ventilated.
Procedure:
- Wear protective gear.
- Set up a temperature probe in a water bath at room temperature. Put 4 mL of 3% H2O2 into a test tube. Place test tube into the water bath. Draw 1 mL of KI solution into a pipette and also place into the water bath.
- Set up the Gas Pressure sensor with the lab quest. Set the rate to 0.1 samples/second. Set time to 300 seconds.
- To begin the reaction, transfer the KI solution in the pipette to the test tube quickly. Cover with the gas pressure sensor stopper, and shake lightly the mix the reagents. Make sure to keep the test tube in the water bath throughout the reaction.
- Start data collection, making sure to keep it collecting for at least 2 minutes. Then remove the stopper and clean out the contents.
- Determine the initial rate of the reaction. After graphing the data, choose a linear portion of it. Choose a linear fit for this data in the analyze menu on logger pro. Record the slope as the initial rate. Save data.
Part II:
- Measure out 4 mL of 3% H2O2 solution in a test tube. Seal and place into the water bath.
- Add 1 mL of distilled water to 1 mL of KI solution, and mix
- Draw 1 mL of the new KI solution into a pipette and place pipette into the water bath.
- Repeat steps 4- 6 in part I. Save data.
Part III:
- Mix 2 mL H2O2 with 2 mL distilled water. Transfer 4 mL of this solution to a test tube, and seal
- Draw 1 mL of .5 M KI solution in a pipette and invert in the water bath.
- Repeat steps 4- 6 in part I and save the data.
Part IV and V:
Conduct the part IV and V identically to the procedure in part I, with one exception: set the water bath to 30 degrees Celsius for the first trial and 40 degrees Celsius for the second trial.
Data:
| Part | Reactants | Temperature (ᵒC) | Initial rate (kPa/s) |
| I | 4 mL 3.0% H2O2 + 1 mL 0.5 M KI | 25 | .3340 |
| II | 4 mL 3.0% H2O2 + 1 mL 0.25 M KI | 25 | .2011 |
| III | 4 mL 1.5% H2O2 + 1 mL 0.5 M KI | 25 | .1719 |
| IV | 4 mL 3.0% H2O2 + 1 mL 0.5 M KI | 34 | .4004 |
| V | 4 mL 3.0% H2O2 + 1 mL 0.5 M KI | 40 | .9935 |
Calculations:
Converting the initial rate from kPa/s to Molarity/s for Part 1f
PV=nRT
P/RT=n/V=mol/liter=Molarity
0.3340/(8.314)(25+273) =M (Ideal gas constant for kPa = 8.314)
1.35×〖10〗^(-4)=M/s (P was in kPa/s; the answer is M/s)
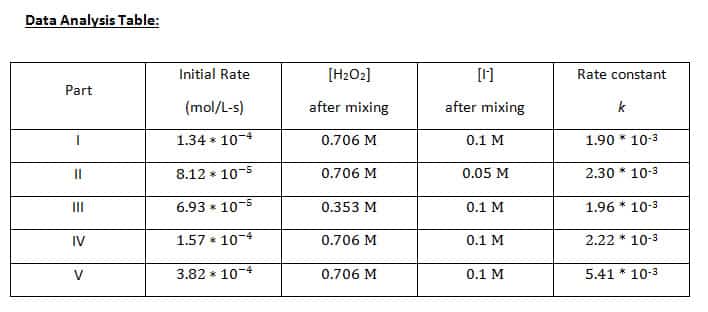
Finding [H2O2] after mixing
3% H2O2 has a concentration of 0.882 M; we used 4 mL + 1 mL of KI
(0.882 M)(4 mL)= 3.53 mmol H2O2
(3.53 mmol H2O2/(5 mL total volume)= .706 M H2O2
Finding [I–] after mixing
We used 1 mL of 0.5 M I– with 4 mL of H2O2
(0.5 M)(1 mL)= 0.5 mmol I^-
(0.5 mmol I^-)/(5 mL total volume)= .1 M I^-
Post-Lab Questions:
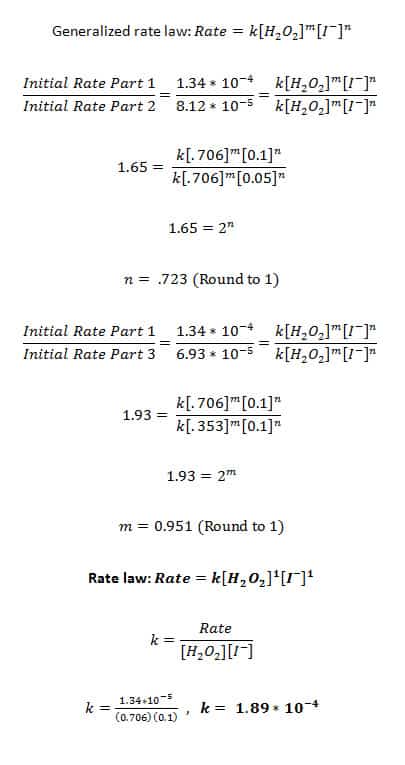
1) Calculate the rate constant and write the rate law expression for the catalyzed decomposition of hydrogen peroxide. Explain how you determined the order of the reaction in H2O2 and KI.
12 The following mechanism has been proposed for this reaction:
H2O2 + I– -> IO– + H2O (Step 1)
H2O2 + IO– -> I– + H2O + O2 (Step 2)
If this mechanism is correct, which step must be the rate-determining step? Explain.
The rate determining step, or slow step, must be step 1. Our rate law expression was determined experimentally to have an order of 1 for both hydrogen peroxide and iodine. Because the coefficients of both reactants in step 1 are one, the order of the proposed rate law of this mechanism would match the order of our determined experimental rate law if the first step was the rate-determining slow step.
I determined the order of the reaction for hydrogen peroxide and KI by first writing a general rate law, where the order of both reactants was unknown. I then divided the initial rate laws for Parts I and II, which canceled out the hydrogen peroxide (as the concentration of hydrogen peroxide did not change in these parts).
I was left with the concentration of iodine ion raised to an unknown power, and taking the log of this expression I was able to find the order for potassium iodide, which turned out to be 1. I then divided Parts I and III and took the log of that expression to find the order of hydrogen peroxide, which was also 1.
Conclusion:
The reaction that occurred during this lab was the decomposition of hydrogen peroxide catalyzed with the presence of potassium iodide. The decomposition of hydrogen peroxide by itself is
2H2O2(aq) -> 2H2O(l) + O2(g).
However, this was not the exact reaction that took place. We added KI to the hydrogen peroxide because KI is a known catalyst and it would speed up the reaction. Catalysts are defined by being substances that increase (or decrease) the rate of a chemical reaction without being consumed in the process. If the proposed mechanism for the reaction between H2O2 and I– is correct, then the iodine ion perfectly fits the definition of a chemical catalyst.
H2O2 + I– -> IO– + H2O (Step 1)
H2O2 + IO– -> I– + H2O + O2 (Step 2)
It can be seen that the iodine ion (provided by the KI solution) is a product as well as a reactant. Therefore, the iodine added to the solution is never consumed; it never interferes with the reaction. The decomposition of hydrogen peroxide is spontaneous; it would occur no matter what over a period of time. Adding iodine, however, considerably speeds up the reaction. Catalysts are incredibly useful and sometimes vital in chemistry because they are able to significantly change the rate of the reaction without interacting with the reaction itself.
One way we can measure the change in the rate of the reaction is by using rate laws. Rate laws are able to quantify the rate at which a reaction occurs; they are the basis of studying the kinetics of chemistry. Rate laws are written in the form , where k is the rate constant and the concentrations of A and B are raised to their coefficient’s power (in elementary processes). The degree of the coefficient tells us the order of the reactant for that particular rate law.
This order signifies how much that reactant affects the rate of the reaction. For example, if the order of the above reaction for A was 2 and the order for B was 1, the reactant A would affect the rate more than the reactant B. We determined experimentally that the order for both H2O2 and I– was 1. This means that the concentration of both reactants affect the rate of the reaction equally. This follows in the chemistry of catalysts, as if the concentration of the catalyst increases, there would be more catalysts to speed up the reaction and the rate would increase.
Our determined rate law was . We found this through comparing several different variations of the catalyzed decomposition of hydrogen peroxide. In Part I, we established the basic parameters that we would use a 0.706 M H2O2 solution and a 0.5 M KI solution. In Part II we halved the molarity of the KI; in Part III we halved the molarity of the H2O2. We then saw how changing the molarity for both reactants affected the initial rate.
(The initial rate was determined to be the slope of the graph of pressure vs. time. This works because the decomposition of H2O2 creates oxygen gas, which would increase the pressure in the test tube over time.) By dividing the initial rates of Parts I and II, we saw how the rate changed when the concentration of I- changed, because the concentration of H2O2 stayed the same. This change was quantified using the general rate law of and solving for m and n. Comparing Parts I and III allowed us to see how the change in concentration of hydrogen peroxide would change the rate of the reaction. In the end, the order of both reactants was found to be 1.
Finding the rate constant was the next step. Because we had already established the rate law for this reaction, we could plug in the values for the Rate, and solve for k for Parts I, II, and III. The rate constant depends on the temperature of the reaction (as higher temperatures would cause the rate to go up without any bearing on the concentration of the reactants), so we could not compare the rate constants of Parts IV and V, as they are at higher temperatures.
However, the rate constants for Parts I, II, and III were 1.90 * 10-3, 2.30 * 10-3, and 1.96 * 10-3, respectively. These values hover reasonably close together, so we are able to say that the rate constant for the catalyzed reaction of hydrogen peroxide at 25 ᵒC is close to the average of the three, around 2.05 * 10-3.
Parts IV and V could be used to find the activation energy of the reaction. Activation energy is the minimum energy to start a chemical reaction. We were able to determine our activation energy by manipulating the Arrhenius equation around to be in the form of y = mx + b. This form is , where ln(k) is y, 1/T is x, and Ea/R is m, the slope of the graph. By multiplying the slope of the graph by a negative R value, we were able to solve for the activation energy.
Error Analysis:
This lab was somewhat free of error, with our rate constant values being relatively constant. The biggest diversion from this value was Part II, which was 2.30 * 10-3. This may have resulted due to a number of reasons. The stopper on the test tube was always popping off in Part II because of the pressure building up inside, so we had to hold the stopper ourselves to get any usable amount of data. This may have caused a change in the pressure readings, which would have changed all of our results significantly.

where is the graph??
how did you get the rate constant k in your table?
good question
2^.723=1.65
How did you get n=.723 from 1.65=2^n?
log base 2 of 1.65