Methyl salicylate C6H4 (HO) COOCH3, also known as salicylic acid methyl ester, oil of wintergreen, is a natural product of many species of plants. Methyl salicylate may be used by plants as a pheromone to warn other plants of pathogens such as tobacco mosaic virus. Methyl salicylate is used in deep heating liniments and in small amounts as a flavoring agent. Oil of wintergreen is used in the field of medicine in aromatherapy.
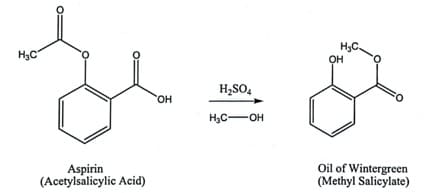
Reaction
Objectives
To synthesize methyl salicylate by esterification of acetylsalicylic acid with methanol.
To separate the synthesized methyl salicylate
Materials
Bottle of aspirin, methanol, concentrated sulfuric acid, sodium bicarbonate, Ether, mortar and pestle, scintillation vials, pipettes, graduate cylinder, hot plate.
Safety
Always wear goggles and gloves Work under fume hood
Do not breathe vapors of methanol and ether
Handle concentrated sulfuric acid with care. If spilled on your skin wash immediately with plenty of water.
Procedure
- Weigh two tablets of aspirin on the analytical balance scale to 0.01 gram.
- Mass = 0.74 g
- Place in mortar and decompose the aspirin to a powder form.
- Pour the powder aspirin into a scintillation vial. Add 5 ml of methanol.
- Add 4 drops of concentrated sulfuric acid to the bottle. Gently swirl so the contents are thoroughly mixed. Be careful the reaction is exothermic.
- Gently warm on hot plate for approximately 5 minutes.
- Cool down and then neutralize the salicylic acid with sodium bicarbonate. Add one drop at a time until the reaction stops fizzing.
- mL needed = 7.60 mL
Extraction
Use ether (or any other solvent available)
Do several extractions to remove by products
For each extraction two layers form.
The top layer is ether and the bottom layer is water/ methanol
Remove top layer with pipette.
Continue to evaporate the ether by a water bath in the fume hood.
After evaporation the smell of methyl salicylate is produced
Conclusion
How many moles of salicylic acid and methanol are used in this experiment?
Salicylic Acid: 0.74 g / 138.121 g/mol = 5.36 x 10^-3 moles
Methanol: 0.005 L x 0.998 mol/L = 4.99 x 10^-3 moles
Draw the two functional groups found in esters?
Esters are made from carboxylic acid and alcohols
R-CO-OR made from R-COOH and R-OH
What is the purpose of concentrated sulfuric acid in an esterification reaction?
The purpose of the sulfuric acid is to act as a catalyst.
In esterification, an ester is formed by the reaction between an alcohol and an organic acid (usually a carboxylic acid). The reaction typically involves the removal of a water molecule (dehydration) to form the ester.
Sulfuric acid acts as a catalyst by protonating the carboxylic acid, generating a more electrophilic carbonyl group. This makes the carbonyl carbon more susceptible to nucleophilic attack by the alcohol. The sulfuric acid can also abstract a water molecule from the reaction mixture, driving the equilibrium towards ester formation.
The dehydration of the reaction mixture is essential because water, which is produced as a byproduct in the esterification reaction, can hydrolyze the ester back into the starting materials. Removing water through dehydration shifts the equilibrium of the reaction in favor of the ester product.
Write the neutralization reaction between sulfuric acid and sodium bicarbonate
H2SO4 + NaHCO₃ -> Na2SO4 + H2O + CO2
Which substance is the limited reagent in the esterification reaction? Explain!
Salicylic acid + Methanol → Methyl Salicylate
C₇H₆O₃ + CH₃OH → C₈H₈O₃ + H2O
The stoichiometric coefficients of each reactant is 1
This means that the reactants will react evenly with each other
Amounts of each substance:
Salicylic Acid: 5.36 x 10^-3 moles
Methanol: 4.99 x 10^-3 moles
Since both of these reactants react at the same rate (x moles of Salicylic Acid reacts with x moles of Methanol), it is evident that Methanol is the limit reactant because it will be fully reacted before all of the Salicylic Acid reacts. It will leave (5.36 x 10^-3 – 4.99 x 10^-3) 3.7 x 10^-4 moles of Salicylic Acid unreacted.
What is the percent yield of methyl salicylate?
Theoretical value: Since Methyl Salicylate is produced at a 1:1 ratio with either of the reactants, the amount of moles of Methyl Salicylate produced will be equal to the moles of limiting reactant available, which in this case is 4.99 x 10^-3 moles.
0.00499 moles x 152.1494 g/mol = 7.59 g
Actual value:
0.0035429 moles of methyl salicylate
0.0035429 moles x 152.1494 g/mol = 5.39 g
Actual Value/Theoretical Value x 100 = % Yield
0.0035429/0.00499 x 100 = 71%
- Why is the percent yield of the product either too high or low?
Evaporated off
- What are the two additional byproducts produced in the synthesis of methyl salicylate?
Acetic acid and water
- What is the purpose of the extraction technique in this lab?
Extract the ester using an ether
