Breathalyzer – Alcohol testing devices
 The Breathalyzer device contains: A system to sample one’s breath and Two glass vials containing the chemical reaction mixture. A system of photocells connected to a meter measure the color change associated with the chemical reaction. To measure alcohol, a person breathes into the device. The breath sample is bubbled in one vial through a mixture of sulfuric acid, potassium dichromate, silver nitrate and water. The silver nitrate is a catalyst, a substance that makes a reaction go faster without participating in it. The sulfuric acid, in addition to removing the alcohol from the air, also might provide the acidic condition needed for this reaction. During this reaction, the reddish-orange dichromate ion changes color to the green chromium ion when it reacts with the alcohol; the degree of the color change is directly related to the level of alcohol in the expelled air. To determine the amount of alcohol in that air, the reacted mixture is compared to a vial of unreacted mixture in the photocell system, which produces an electric current that causes the needle in the meter to move from its resting place. The operator then rotates a knob to bring the needle back to the resting place and reads the level of alcohol from the knob — the more the operator must turn the knob to return it to rest, the greater the level of alcohol.
The Breathalyzer device contains: A system to sample one’s breath and Two glass vials containing the chemical reaction mixture. A system of photocells connected to a meter measure the color change associated with the chemical reaction. To measure alcohol, a person breathes into the device. The breath sample is bubbled in one vial through a mixture of sulfuric acid, potassium dichromate, silver nitrate and water. The silver nitrate is a catalyst, a substance that makes a reaction go faster without participating in it. The sulfuric acid, in addition to removing the alcohol from the air, also might provide the acidic condition needed for this reaction. During this reaction, the reddish-orange dichromate ion changes color to the green chromium ion when it reacts with the alcohol; the degree of the color change is directly related to the level of alcohol in the expelled air. To determine the amount of alcohol in that air, the reacted mixture is compared to a vial of unreacted mixture in the photocell system, which produces an electric current that causes the needle in the meter to move from its resting place. The operator then rotates a knob to bring the needle back to the resting place and reads the level of alcohol from the knob — the more the operator must turn the knob to return it to rest, the greater the level of alcohol.
Head Space Gas Chromatography- Testing Alcohol levels in Blood and Urine
The ‘headspace’ is the gas space in a chromatography vial above the sample. Headspace analysis is therefore the analysis of the components present in that gas.Headspace GC is used for the analysis of volatiles, like ethanol samples.
Phases of the Headspace Vial
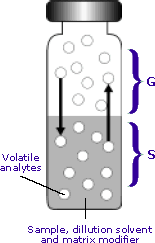 G = the gas phase (headspace)
G = the gas phase (headspace)
The gas phase is commonly referred to as the headspace and lies above the condensed sample phase.
S = the sample phase
The sample phase contains the compound(s) of interest. It is usually in the form of a liquid or solid in combination with a dilution solvent or a matrix modifier.
Once the sample phase is introduced into the vial and the vial is sealed, volatile components diffuse into the gas phase until the headspace has reached a state of equilibrium as depicted by the arrows. The sample is then taken from the headspace.
Infrared Technology
Current technology uses infrared measurement systems that are made more specific for alcohol by using several optical filters. You determine breath alcohol levels by passing a narrow band of IR light, selected for its absorption by alcohol, through one side of a breath sample chamber. By detecting emergent light on the other side, you can measure alcohol concentration by using the well-known Lambert-Beers law, which defines the relationship between concentration and IR absorption.
Fuel Cell Technology
Alcohol fuel cell consists of a porous, chemically inert layer coated on both sides with finely divided platinum (called platinum black). The manufacturer impregnates the porous layer with an acidic electrolyte solution, and applies platinum wire electrical connections to the platinum black surfaces. The manufacturer mounts the entire assembly in a plastic case, which also includes a gas inlet that allows a breath sample to be introduced. Various manufacturers employ numerous proprietary nuances in their construction.
