
An ideal gas is a theoretical gas that perfectly fits into the equation PV= nRT. An ideal gas is different from a real gas in many ways.
Ideal gases abide by all gas laws regardless of the pressure of temperature; however in reality they do not exist, hence the terminology “ideal”.
They occupy no volume, unlike real gases which occupy small volumes. Ideal gas particles exert no attractive forces; their collisions are purely elastic. Real gases excerpt small attractive forces.
The pressure of an ideal gas is much greater than that of a real gas since its particles lack the attractive forces which hold the particles back when they collide. The differences between ideal gases and real gases can be seen most evidently when the pressure is high causing the gas particles to occupy a smaller volume or when the temperature is low, causing lower kinetic energy.
The difference can also be more apparent when the gas particles are larger, and when the gas particles excerpt strong attractive forces. Monoatomic gas molecules are much closer to ideal gases than other particles since their particles are so small. Because of the differences between ideal and real gases, Van der Waals created an equation to relate the two:
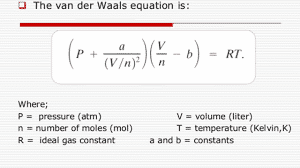
Where a and b represent the strength of the intermolecular attraction and excluded volume (These vary with each particular gas).

An ideal gas is a hypothetical gas or one which isn’t available in nature?
Why does this matter?
what do you mean