INTRODUCTION
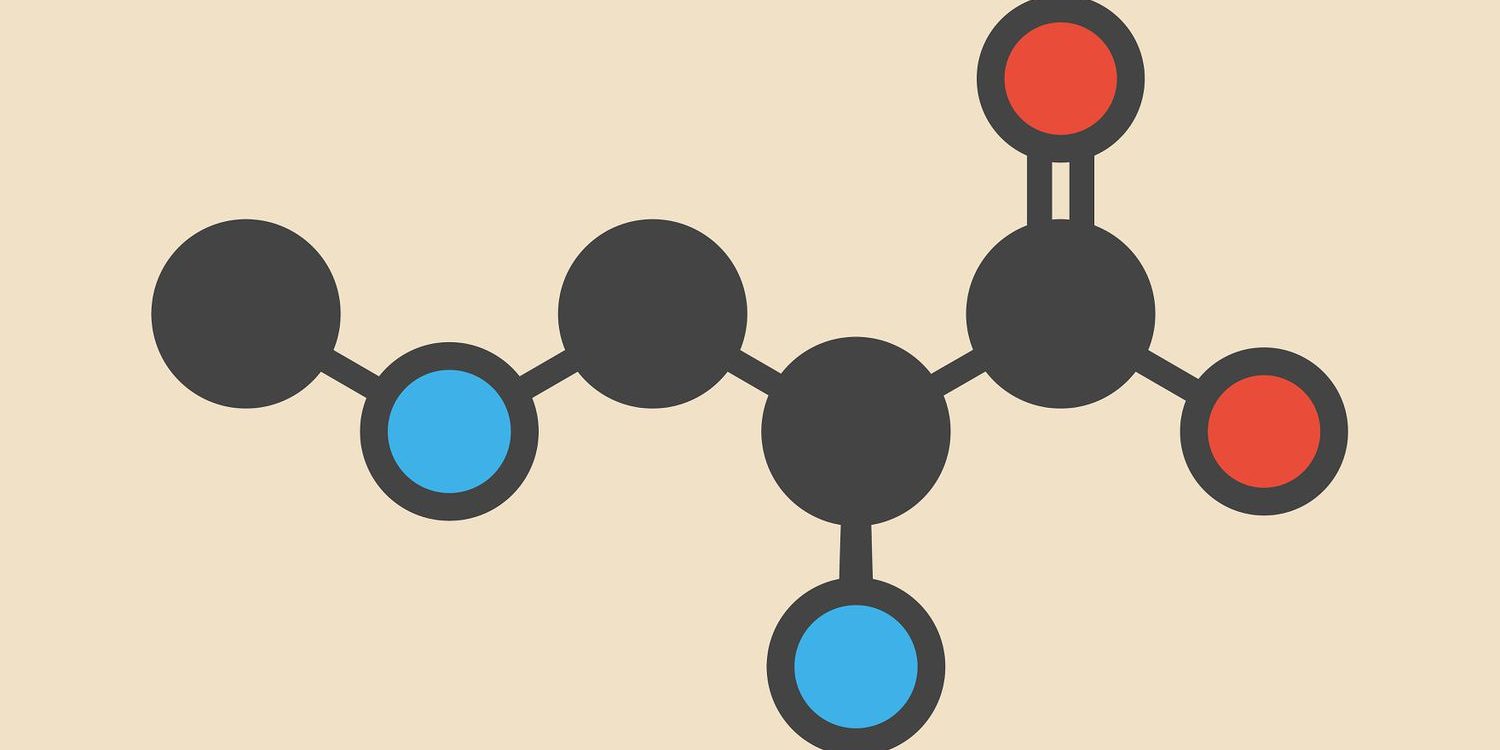
Sodium polymethacrylate is an example of a condensation polymer. On its own, it is tightly wound and appears like a granule of sugar. The monomer of sodium polymethacrylate is shown below.
When water is added, the polymethacrylate will absorb the water through osmosis. As a result, hydrogen bonds with the oxygen in water. As this occurs, the polymethacrylate molecule will unwind and become larger. Sodium polymethacrylate can absorb up to 200-300 times its mass in water.
PURPOSE
To explore polymerization by dissecting a baby’s diaper and to determine how polymers are employed to keep a baby dry.
MATERIALS
- Baby powder/cornstarch
- Scissors
- Sodium chloride
- 100 mL beakers
- Sugar
- Paper
- Water
- Disposable baby diaper
- Scale
SAFETY PRECAUTIONS
- DO NOT BREATHE IN THE CRYSTALS OR TOUCH YOUR FACE/EYES AFTER COMING INTO CONTACT WITH THEM.
- WASH YOUR HANDS AFTER COMPLETING
- THIS LAB OR WEAR GLOVES PROVIDED!
PROCEDURE
- Get a diaper for you and your partner.
- Cut the disposable diaper into 2 pieces widthwise.
- Shake the contents of each half of the diaper out onto a sheet of paper.
- Place the materials from the inside of the diaper into a beaker.
- Gently shake the beaker back and forth.
- Remove the ball of cotton that rises to the top of the powder collected and make observations about the substance left.
- After removing the ball of cotton that rose to the top of the powder, the remaining substance has a crystal/powdery texture. It looks like fine crystals or powder. After removing the cotton ball, there is very little powder remaining. The cotton ball mixed and quickly separated from the crystals or powder, suggesting that the two objects have different physical qualities.
- Add 100 mL of water to the powder from the inside of the beaker and stir.
- After putting in the substance from the diaper into the water and shaking it, a white solid started to form. It looks like ice, however, it is a jelly-like substance with a cotton substance to it. It is clear/whitish. It is fluffy and has a powdery texture and moves like putty.
- Record any observations.
- Divide the mixture into 3 portions using the remaining 2 beakers.
- Prepare a table to record your observations as you complete steps #11-14.
- To one portion of the mixture, add 2 mL of sugar and record any observable changes.
- To another portion of the mixture add 2 mL of sodium chloride and record any changes seen.
- To the final portion add 2 mL of baby powder and record any changes.
- Cut a 2 cm x 2 cm sample of the following parts from the diaper and describe the properties of these materials. Attach these samples to your rough data table (Partners will each have the originals or one can take photos of the samples to hand in).
Parts: Outer back sheet, inside the top sheet, adhesive tape, elastic around leg cuffs, fluffy filling
- Pour the 3 samples into the remaining diaper pieces and put the diaper into the garbage.

| Steps | Observations |
| 11 | When 2 mL of sugar was added to the mixture, the texture became less fluffy, and the substance seemed to gather or combine instead of remaining in little fragments. The colour remained constant, with no major changes noticed and the sugar simply dissolved into the mixture. |
| 12 | A dramatic change occurred after adding 2 mL of sodium chloride to the liquid. All obvious chunks disappeared as the mixture melted. The mixture’s volume shrank, leaving behind small specks or particles hanging in the liquid. As a result, the final substance was translucent, with a clear or slightly hazy appearance as a liquid. |
| 13 | The mixture changed significantly after 2 mL of corn starch was added. It got puffy and expanded in volume, resulting in an increase in overall size. The texture became hazy, with a frothy and foamy quality. The colour changed to a milky white tint. When handled, the substance broke down and acquired a waxy or candle-like texture. |
| 14 | The diaper’s outer back sheet is made of a soft, thin paper substance. It has a felt-like texture that provides a soft touch. The inside top sheet of the diaper has fluffy filled circles that look like bubble wrap. This layer is substantially thicker than the outer back sheet, resulting in improved absorption and comfort. The diaper’s adhesive tape is firm but flexible, allowing it to efficiently secure the diaper in place. The elastic material utilised around the diaper’s leg cuffs is stretchy, soft, and flexible. It provides a secure fit while enabling the infant to move freely. The fluffy filling inside the diaper feels like cotton. It is velvety and dense, with good absorbency. It is not easily stretchy or pliable (not ductile) and retains its shape. |
Do not dispose of any materials in the sink!
Answer the questions that follow in full and complete sentences (typed). Hand them in with your data/observations.
ANALYSIS & APPLICATION
- Based upon the structure of sodium polymethacrylate and your knowledge of intermolecular forces, polarity, and organic chemistry, explain the changes observed to the crystals when water was added.
Methacrylic acid, a monomer, undergoes a transformation called polymerization to produce sodium polymethacrylate, a larger structure composed of interconnected units. The ionisation of methacrylic acid generates negatively charged carboxylate groups, which facilitate the incorporation of sodium ions into the polymer matrix, ensuring electrical equilibrium. Due to the intermolecular interactions, polarity, and organic chemistry of the polymer, many modifications take place when water is added to sodium polymethacrylate crystals:
As for the oxygen and hydrogen atoms having different levels of electronegativity, water molecules are strongly polar. In water, the hydrogen atoms have partial positive charges while the oxygen atoms have partial negative charges (HYDROGEN BONDING). The sodium polymethacrylate polymer’s polar functional groups, such as the carboxylate groups or any lingering unreacted methacrylic acid units, can establish hydrogen bonds with the water molecules.
The polymer dissolves in water as a result of this interaction, which breaks up the polymer’s crystal lattice. Positive sodium ions interact with the negative oxygen atoms in water to form ion-dipole interactions (DIPOLE-DIPOLE). This is made possible by the presence of sodium ions in sodium polymethacrylate. By helping to separate the polymer chains and decreasing the intermolecular forces holding the crystal lattice together, this interaction improves the polymer’s ability to dissolve in water.
Water molecules surround and solvate the polymer segments as the polymer chains dissolve in the liquid. The hydration of polymers is the process in question. Due to this hydration, the crystals of sodium polymethacrylate can swell or increase in size. Water molecules’ polarity enables them to interact with the charged and polar functional groups found in polymer chains, facilitating the solvation and dispersion of the polymer chains in the aqueous solution (POLYMERIZATION).
- Based upon the structure of sodium polymethacrylate and your knowledge of intermolecular forces, polarity, and organic chemistry, explain the changes seen to the mixture with the addition of sugar, sodium chloride and corn starch.
Sodium polymethacrylate is a polymer composed of repeating units of the monomer methacrylic acid, which is modified with sodium hydroxide to make it water-soluble.
With the addition of sugar, the changes seen to the mixture are reasoned by sugar’s hydrophilic nature which made it dissolve in the water. The polar hydroxyl groups present in sugar molecules can form hydrogen bonds with water molecules, and since sodium polymethacrylate is also water-soluble, the sugar will likely mix uniformly with the polymer solution. This means that the change in texture can be justified by the water simply being dissolved with the crystalline structures of sugar causing the reduction in fluffiness, increase in viscosity, and more of a semi-solid feel when mixing the mixture.
The addition of sodium chloride liquified the mixture with small particles hanging around in the liquid. Adding the sodium chloride to the sodium polymethacrylate and water solution, the sodium and chloride ions separate and become solvated by the water molecules in the mixture. The presence of the chloride ions may also disrupt the interactions between the polymer chains of sodium polymethacrylate, potentially causing the polymer to break down or precipitate as well. This explains the liquified mixture and the particles still present as well.
The addition of sodium chloride to the sodium polymethacrylate and water solution causes the mixture to become more liquid, with suspended particles. This was observed in the lab as the mixture liquified and left behind a translucent liquid. This occurs as the sodium and chloride ions separate and solvate with the water molecules, while also disrupting the interactions between the polymer chains of sodium polymethacrylate. Consequently, the polymer may break down or precipitate, resulting in the liquified nature of the mixture and the presence of remaining particles, which can explain the little pieces floating around in the liquid.
Upon the introduction of corn starch to the mixture, the volume of the mixture increased also leading to a gelatinized product. Corn starch is a complex carbohydrate consisting of long chains of glucose molecules which, upon addition to the mixture, corn starch particles disperse in the solvent, which is water in the case of sodium polymethacrylate. The hydrophilic nature of corn starch allows it to form hydrogen bonds with water molecules, leading to the starch’s expansion in volume or swelling and gelatinization.
Including corn starch in the mixture modifies its texture and thickness, resulting in a noticeable waxy sensation when stirring. This is due to the gelatinized starch acting as a thickening agent and increasing the overall viscosity of the mixture. The overly viscous mixture may be a reason as to why it broke down when stirred. The addition of corn starch can affect the stability of the sodium polymethacrylate solution. The presence of starch particles can interfere with the interactions between the polymer chains, leading to the formation of aggregates or precipitates. This explains why there was froth formed on the top of the reaction mixture.
- The properties of sodium polymethacrylate are ideally suited for its use in diapers and feminine hygiene products. Suggest at least 3 other uses for this polymer.
Sodium Polymethacrylate is one of the most important things as its function is what makes a diaper usage effective. This chemical is known to absorb the liquids that are put into the diaper, this is used to pull the liquid away from the skin itself. Therefore this is an absorbent polymer.
Some other usages of sodium polymethacrylate are skin conditioners, a hair fixative or a film former. This polymer is very strong as well as flexible, allowing the molecule to absorb and gel which locks into water. With this effect, it can be used to keep the skin products’ texture conditioned. For hair fixatives, it serves a great purpose and is essential for cleansing hair, as it is mainly used for health care because its properties allow it to remove cationic build-up on hair. Making it ideal for usage in shampoos and various other hair care products. It also provides moisture, texture and glossy shine for hair. The final product you can find is sodium polymethacrylate within film former. Film former is a hair and skin care product that is responsible for moisturization. As its water bonding is used on the skin with the absorption help from the polymer.
- Show the condensation reaction that results when the monomer(s) reacts to form polymethacrylate.
In a condensation reaction, two or more molecules combine to form a single larger molecule. As a result of this, a small molecule, such as water, carbon dioxide or alcohol is usually eliminated during a condensation reaction. Condensation polymers form more slowly than addition polymers, often requiring heat and generally lower in molecular weight. The terminal functional groups on a chain remain active, so that groups of shorter chains combine into longer chains in the late stages of polymerization.
In the following reaction, the monomers, methanol and methyl acrylate result in the byproducts of polymethacrylate and water. Water (H2O) is the small molecule that is removed in the reaction. This occurs when a methyl acrylate molecule’s (-H) molecules interact with a methanol molecule, (-OH) molecules react with each other to form H2O. The 2 monomers are mixed together at high temperatures to form polymethacrylate. Since the molecule isn’t a radical, cation or anion, this is no termination step. The reaction just ends when the conditions aren’t favourable or there is no more stuff to react.
n(CH3OH) + n(C4H6O2) → (C5O2H8)n + 2nH2O
- Polymers are often thought to be manufactured in immense chemical plants. Although many are, there is a large group of polymers which are natural (biopolymers). Describe several natural polymers and how they are utilised in our society or in our bodies. Pick one and show its reaction.
There are many natural polymers such as cellulose, nucleic acids, proteins, carbohydrates, rubber, chitin, silk and wool and they are widely utilised in our society and our bodies.
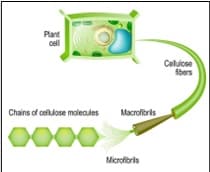
Cellulose – A natural polymer, found in plants cell walls is cellulose. The image to the right shows how chains of cellulose molecules ultimately support the cell wall of plants. It is actually the most common organic material because it is obtained mostly from plant biomass including cotton, wood pulp, and other plant fibres. Cellulose fibres are collected from the mentioned sources and are then shaped into a variety of forms of the consumer’s choice. In our society – cellulose is widely used to make paper and cardboard. For example, after being extracted from wood pulp, the cellulose fibres are made into sheets.
These sheets are then converted to cardboard, paper products and everyday packaging, which are ultimately used in our everyday packaging, writing, and so on. There are also cotton plant fibres that are also used in making other cellulose-based textiles, such as cotton and rayon. In fact, cellulose is sometimes used to make major biofuels and other renewable resources we use. And lastly, our bodies also use cellulose to aid with digestion and help maintain a regular and consistent process. In our large intestine, the cellulose ferments with other fibres, making fatty acids in our bodies. Then, these fatty acids are used provide energy to the cells that line the colon and these provide many other health advantages.
Nucleic acids – Other major polymers that we must need are nucleic acids, such as DNA and RNA. These nucleic acids are actually produced among all living organisms, including us. These acids are obtained from cells and tissues and other biological sources. We create these acids, which cells use to control the normal cellular activities. For example, DNA comes from the core/nucleus of our cells. The DNA molecule carries the genetic code and acts as a blueprint which determines an organism’s characteristics and qualities. Next, through transcription, DNA converts to RNA, which is needed for protein synthesis, gene regulation, and many other biological functions.
Since these nucleic acids hold such high value, they are widely used in our society. Firstly, they are used in the biotechnology and genetic engineering field because scientists can manipulate our DNA and RNA to study genes and understand disorders. This leads to new therapies and another diagnosis of illnesses. These are also used in crime investigation as samples collected at a crime scene can be compared to suspect profiles. And at last, it is used in the medicine field to detect possible disorders or diseases, and develop potential drugs.
Proteins – Proteins are macromolecules made of amino acids that are bonded by peptide bonds. The peptide bonds share the purpose to bond amino acids and proteins. Proteins are found in various parts, including animal and plant tissues, microbes, and synthetic sources. These proteins are used to provide our bodies with structural support to cells and tissues, enzymes (which work as catalysts to speed up processes in our bodies), hormone production/regulation, immune system strengthening, and overall cell communication. They are also used in diet and widely benefit the economy.
For example, people nowadays prefer plant-based proteins (soybeans, legumes, beyond meat) due to their unique texture, as a protein-high food as our bodies require them and overall as food flavour enhancers. Lastly, they are used in the medical industry because of their ability to perform in tissue engineering, vaccines, medicines production and research and other diagnostic instruments.
Rubber – Rubber is another naturally occurring polymer obtained mostly from the latex of numerous plants, most notably the rubber tree (Hevea brasiliensis). When an incision in the bark is made to collect the milky sap containing rubber particles, the latex is taken from the tree. Rubber has unique characteristics such as flexibility, durability, and waterproofness. It is used in everyday life, including footwear, gloves, balloons, conveyor belts, toys, and many more items.
Carbohydrate – Another major natural polymer is the carbohydrate molecule, which is made of oxygen, carbon and hydrogen atoms. These are usually found in plant-based foods, mainly grains, fruits and vegetables. Our bodies surely require them as a source of energy as these are turned into glucose as we digest foods with carbohydrates. Our cells then use that glucose to create ATP, the primary energy source for all living organisms to function, and eventually perform all daily tasks. These carbs can also be stored in our muscles and liver, for when energy is needed again. Similarly to proteins, they have a food economy due to consumers wanting these foods due to their texture, flavour and their nutritional value. Finally, they are employed in a variety of industrial processes to create biofuels, chemicals, and medicinal medications.
Chitin – Chitin can be present in fungi’s outside cell walls as well as the outer shells of insects and crustaceans like lobsters and shrimp. It has numerous applications in our society due to its antibacterial properties and ability to produce biodegradable goods. It is used in food packaging plastics, cosmetics, water treatment, and the production of different textiles. It is also used in the medical industry for making scaffolds, used in tissue engineering as wound dressings, and medical sutures.
– The Reaction of Cellulose –
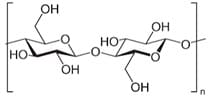
Cellulose is a carbohydrate which is made of repeated glucose chains which are bonded by glycosidic bonds The figure on the right shows the linear chains of glucose. They are ether bonds which connect a carbohydrate (sugar) molecule to another group. Something that makes cellulose a unique polymer is its ability to undergo various reactions.
Hydrolysis – The breaking of the glycosidic bonds that connect the glucose units in the cellulose polymer occurs during the hydrolysis reaction. It is shown as:
(C6H10O5)n + nH2O → nC6H12O6 or cellulose + Water -> Glucose.
The water/H2O molecule has the purpose to break the glycosidic bonds. When it is successful in doing so, the reaction proceeds, which leaves us with the products of glucose molecules. This hydrolysis takes place in the presence of cellulases. These are enzymes which catalyse the bond breakdown and speed up the reaction.
Combustion – Another reaction that cellulose may undergo is combustion, however, this may occur when cellulose is heated to high temperatures with the presence of oxygen. Cellulose has the same chemical formulation as the sugar of C6H12O6. So, when combined with the oxygen we have the balanced equation of:
(C6H10O5)n + 6nO2 → 6nCO2 + 5nH2O or Cellulose + Oxygen -> Carbon Dioxide + Water + (energy).
The combustion reaction generates energy in the form of heat and light. This explains why cellulose-containing materials like wood can be used as fuel.
Thermolysis – Another reaction that cellulose undergoes is thermolysis/pyrolysis, however, this only occurs when temperatures are above 350°C. This causes the breakdown of cellulose due to the absence of oxygen at these temperatures. Since the cellulose is being heated to such hot temperatures, the heat will form additional energy. Hence, the energy will break the bonds within the structure of cellulose. Overall, decomposition will result in the formation of smaller molecules and the formation of products such as bio-oil, gases, and char. This can be shown as
Cellulose ⟶ Bio-oil + Gases + Char
Essentially bio-oil is just a mixture of oxygenated organic compounds. Whereas, the gases produced are different volatile organic compounds, including methane (CH4) or carbon monoxide (CO) and many others. At last, char is some solid residue which remains after the reaction is completed, mainly consisting of ash.
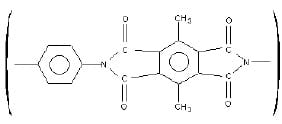
- The structure given below is a polymer classified as a polyamide. This polymer is stable in temperatures above 350°C, and can be used as a protective coating on hot surfaces. What monomers were used to make this polyamide? What polymerization reaction likely took place to create it? Show this reaction.
This polyamide consists of a peptide bond, which is used to bond two smaller monomers together in order to create a polymer. The polymerization reaction which likely took place is a polycondensation. In this case, we are going to see a reaction between an amine and a carboxyl group. Starting from the left side, we see a benzene ring which is simply going to be attached to an amine. The right side is another benzene ring and four carboxylic acid groups.
- 1, 4 – diamino – benzene
- 3, 6 – dimethyl – 1, 2, 4, 5 benzenetetracarboxylic acid
The following are two monomers which were used to make this polyamide:
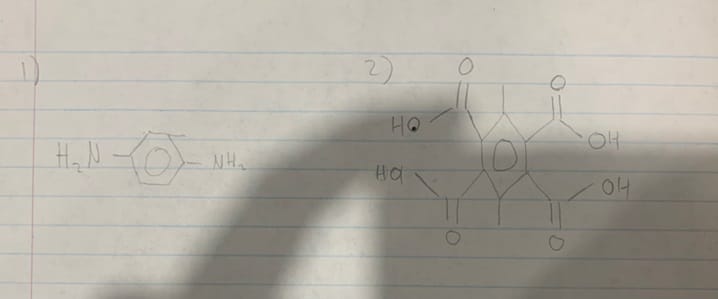
- Amino monomer with two amino groups
- Carboxyl monomer with four carboxylic acid groups
The full reaction is as follows:
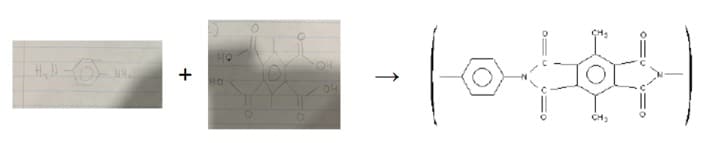
References
Chemical and Biomolecular Engineering at Carnegie Mellon University. (n.d.). Retrieved June 6, 2023, from https://www.cmu.edu/gelfand/lgc-educational-media/polymers/natural-synthetic-polymers/index.html#:~:text=Examples%20of%20naturally%20occurring%20polymers,mentioned%20vulcanized%20rubber%20and%20pectin
eLS – Encyclopedia of Life Sciences. (n.d.). Natural Polymer. In ScienceDirect. Retrieved June 6, 2023, from https://www.sciencedirect.com/topics/materials-science/natural-polymer
Gawande, S. S., & Shetge, A. D. (2020). Biodegradable Natural Polymer. In National Center for Biotechnology Information. Retrieved June 6, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7032228/#:~:text=Cellulose%20is%20a%20highly%20hydrophilic,of%2040%25%E2%80%9360%25
Hancock, E. (1949). The Conformation of Proteins in Solution. In Wiley Online Library. Retrieved June 6, 2023, from https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1478-4408.1949.tb02594.x
Pennsylvania State University. (n.d.). Natural Polymers. In e-Education. Retrieved June 6, 2023, from https://www.e-education.psu.edu/egee439/node/669
Shahidi, F., Arachchi, J. K. V., & Jeon, Y. J. (2010). Food Applications of Chitin and Chitosan. In National Center for Biotechnology Information. Retrieved June 6, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2885078/#:~:text=Chitin%20and%20chitosan%20are%20natural,food%20protection%20and%20nutraceutical%20applications
Structures of Biological Molecules. (n.d.). Retrieved June 6, 2023, from https://chem.libretexts.org/Courses/Saint_Marys_College_Notre_Dame_IN/CHEM_342%3A_Bio-inorganic_Chemistry/Readings/Chapter_5%3A_Biological_Molecules/Structures_of_Biological_Molecules/D._Nucleic_Acids%3A_DNA_and_RNA
The Macrogalleria – Polymers in Sports and Kids. (n.d.). Retrieved June 6, 2023, from https://pslc.ws/macrog/kidsmac/protein.html