Phosphates may be created by substituting some or all of the hydrogen of a phosphoric acid by metals. Depending on the number of 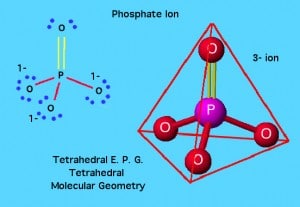 hydrogen atoms that are replaced, the resulting compound is described as a primary, secondary or tertiary phosphate. Primary and secondary phosphates contain hydrogen and are acid salts. Secondary and tertiary phosphates, with the exception of those of sodium, potassium and
hydrogen atoms that are replaced, the resulting compound is described as a primary, secondary or tertiary phosphate. Primary and secondary phosphates contain hydrogen and are acid salts. Secondary and tertiary phosphates, with the exception of those of sodium, potassium and
ammonium are insoluble in water. Tertiary sodium phosphate is valuable as a detergent and water softener. The primary phosphates tend to be more soluble.
Phosphates, which are an important component to metabolism in both plants and animals, help in the first step in oxidation of glucose in the body. Primary calcium phosphate is an ingredient of plant fertilizer.
Phosphates have caused increasing attention recently. The focus is on the environmentally harmful effects in household detergents. Wastewater, from laundering agents, contains phosphates, which are said to be a water pollutant.
Most laundry detergents contain approximately 35% to 75% sodium
triphosphate (Na5P3O10), which serves two purposes. Providing an alkaline solution (pH 9.0 to 10.5) is necessary for effective cleansing and also to tie up calcium and magnesium ions found in natural waters and prevent them from interfering with the cleansing role of the detergent.
Eutrophication is the progressive over-fertilization of water, in which festering masses of algae’s blooms, choking rivers and lakes.  Phosphorus compounds act as a fertilizer for all plant life, whether free-floating algae or more substantial rooted weeds, and are implicated in eutrophication. Many countries control phosphate levels, whereas
Phosphorus compounds act as a fertilizer for all plant life, whether free-floating algae or more substantial rooted weeds, and are implicated in eutrophication. Many countries control phosphate levels, whereas
Switzerland has banned the use of phosphates.
The marine environment is both fragile and more resistant than the terrestrial ecosystem. It is fragile for the reasons that nutrients are generally present in very low concentrations, permanently consumed by living organisms and pollutants diffuse rapidly.
Lakes and rivers are extremely complex ecosystems. Nutrients are taken up by both algae and rooted weeds. The weeds act as a shelter for fish larvae and zooplankton, both of which eat algae and are, in turn, eaten by larger fish. Scientists have concluded that unpolluted lakes can absorb surprisingly large amounts of phosphates without uncertainty. When a fertilizer, such as a phosphate, is added more algae will grow,
and consequently will the populations of zooplankton and fish. Difficulties only arise when the lake is already impure. Zooplankton are sensitive to their environment and many substances are toxic to them. If any of these substances, including phosphates, are present the zooplankton population cannot increase. Adding phosphates to this polluted system will case algae growth. The floating masses cut off the light supply. Weeds die and decompose using up dissolved oxygen, and causing sulfurous smells and plagues. Deprived of shelter and food, the fish larvae starve. The lake is well on the way to catastrophe.
Without wetlands there would be a minimal amount of fresh drinking water due to the fact that wetlands filter the waters of our lakes, rivers and streams, sequentially reducing contamination of water. The plant growth in wetlands removes phosphates and other plant nutrients washed in from the surrounding soil, consequently restricting the growth of algae and aquatic weeds. This growth is a serious problem in some of Canada’s major waterways, where dead and decaying algae deprive the deeper waters of their oxygen.
Researchers at Lancaster University have studied lakes whose plant and animal life has been killed by acid rain. The excess acid in  the lakes can be neutralized easily by adding lime, but this makes the waters rich in calcium. Life will gradually return to the lake but, as these lakes should have low calcium levels, it will not be the same kind of life that existed in lakes before pollution. The answer, they have concluded, is to add phosphates.
the lakes can be neutralized easily by adding lime, but this makes the waters rich in calcium. Life will gradually return to the lake but, as these lakes should have low calcium levels, it will not be the same kind of life that existed in lakes before pollution. The answer, they have concluded, is to add phosphates.
These phosphates work by shielding the water. This depends upon nitrate ions in the lake. Contradictory, these ions also are produced by acid rain, contain oxides of nitrogen from combustion sources. These fertilizers do not alter the pH level of the water. Instead, they stimulate the growth of plants. The plants absorb the dissolved nitrates, generating hydroxide ions, which in return neutralize the excess acid.
Removal of phosphates from detergent is not likely to slow algae growth in containing substances. It may actually prove disastrous. Its replacement with borax will definitely be disastrous. Scientists are unsure of borax role in plant growth. It is not required by algae and other micro plants, but it is essential to higher plants. However in excessive quantities, about 5 micrograms of boron per gram of water, boron severely damages plant life. Highly alkaline substances, gel proteins and sodium hydroxide is hazardous substances. Another concern is the fact that each year thousands of children swallow detergents resulting in serious injuries or death.
In conclusion, the only way to overcome the disastrous effects of phosphates is to find an alternate. However, an acceptable substitute for phosphates has not yet been found. Washing only with synthetic detergents would require so much detergent that the cost per wash would increase significantly. Another alternative is the substitution of synthetic nonionic detergents for ionic detergents in use. Nonionic detergents are not precipitated by Calcium of Magnesium ions. This would reduce the risk contaminating our lakes and rivers.
