Chemical Basis of Cellular Respiration
- Allows for the extraction of energy from sugars (i.e glucose) by slowing oxidizing it
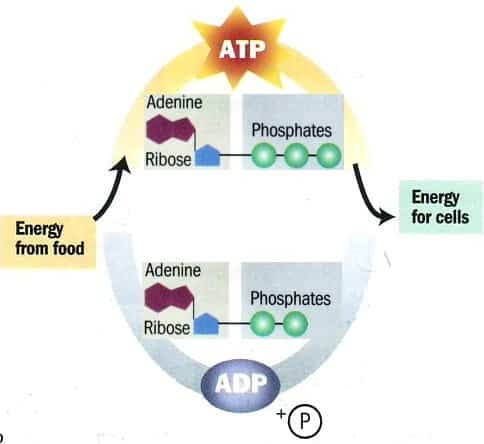
- This process converts potential chemical energy into ATP (which can be used by a majority of reactions)
- C-H bonds (those found in glucose) are the primary energy bond found in organic molecules (i.e glucose, octane etc.)
- Neither Carbon of Hydrogen is electronegative; therefore the electron pair they share can be easily excited and removed
- However the addition of oxygen (highly electronegative) requires more energy input in order to free the electron
- This explains why fats (all C-H bonds) have more energy (calories) per gram than proteins or carbohydrates
- The potential energy released a organic fuel molecule is done in an oxidation reaction
- Oxidation reactions is the loss of electrons (e-), leaving the molecule oxidized
- The oxidation of one reaction is always linked to the reduction of another molecule (gaining an e-)
- In biological organisms, the coupling to oxidation and reduction reactions always occurs (Oxygen usually the oxidizing agent)
- However: in cellular respiration some reactions do not use oxygen as an oxidizing agent; also sometimes the electron transfer is during oxidation can be incomplete!
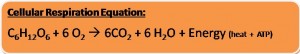
- Like gasoline, glucose undergoes a form of combustion releasing CO2, Water and energy as ATP and Heat.
- The glucose molecule will be fully oxidized and this will result in no energy in the CO2 molecule
- Cellular Respiration is a controlled combustion; the energy in the C-H bonds are released slowly in small catalyst driven reactions
- Both the burning of a glucose molecule and the slow oxidation of it via cellular respiration would yield the same amount of energy. (686 kcal/mol) [EXOGERGONIC]
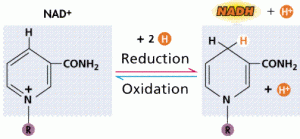
- The Most common carrier of this energy is Nictinamide Adenine Dinucleotide (NADH)
- The enzyme dehydrogenase removes two hydrogen atoms from a substrate molecule and transfers the two electrons and a proton to make the reduction of NAD+ -> NADH
- The energy transfer between NAD and food molecules is highly effective; little energy is loss as heat
Cellular Respiration: Overview
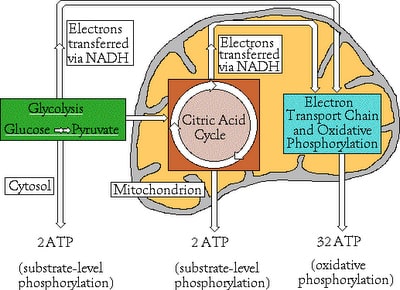
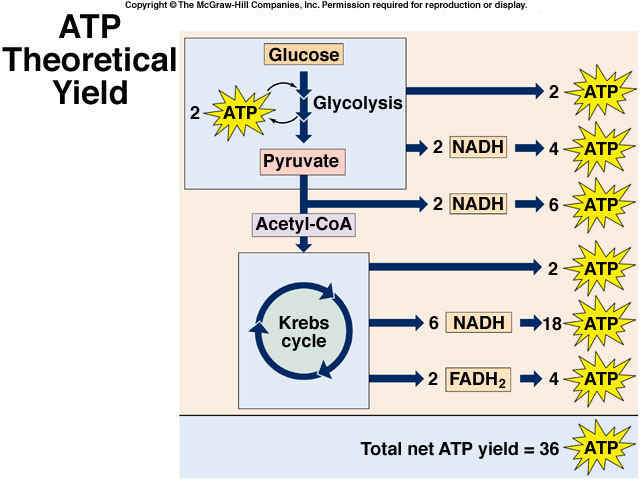
- Consists of three stages: Glycolysis, Citric Acid Cycle (Kerbs Cycle), Electron Transport Chain (ETC)
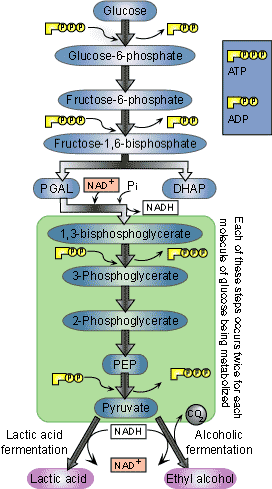
- Glycolysis: Enzymes break down Glucose (6C) into two molecules of Pyruvate (ATP and NADH formed)
- Citric Acid Cycle: Acetyl Co-A from Pyruvate decarboxylation (releases carbon dioxide), enter the Citric Acid Cycle where is completely oxidized to CO2; ATP, NADH, FADH2 are made.
- ETC: NADH and FADH are oxidized; liberating electrons and passing them alone the electron transport chain until they are given to Oxygen and Water is the byproduct. Along the process, free energy moves proton into the inner mitochondrion space where a gradient is established for ATP synthesis.
The Mitochondria
- This membrane bound organelle is the powerhouse of cellular respiration (Largest generator is ATP)
- It has a double-bound membrane; in the matrix the citric cycle and ETC are found
- Its two membranes establish the gradient required to make a majority of the ATP via ATP synthesis
- Only found in eukaryotes; prokaryotes use different mannerisms
Glycolysis
- Oldest of the metabolic pathways (consists of 16 catalyzed reactions)
- 1 Glucose -> 2 Pyruvate
- Glycolysis is universal; found in both eukaryotes and prokaryotes
- It does NOT require O2 (anaerobic) and occurs in the cytosol
- Reactants: 1 Glucose (2 ATP) for phosphorylation
- Products: 4 ATP, 2 NADH (4ATP- 2ATP= 2ATP made overall) , 2 Pyruvate (3C) molecules
- Note*: ATP is made in Glycolysis II via substrate phosphorylation (direct addition of “P” two a substrate group to make ATP)
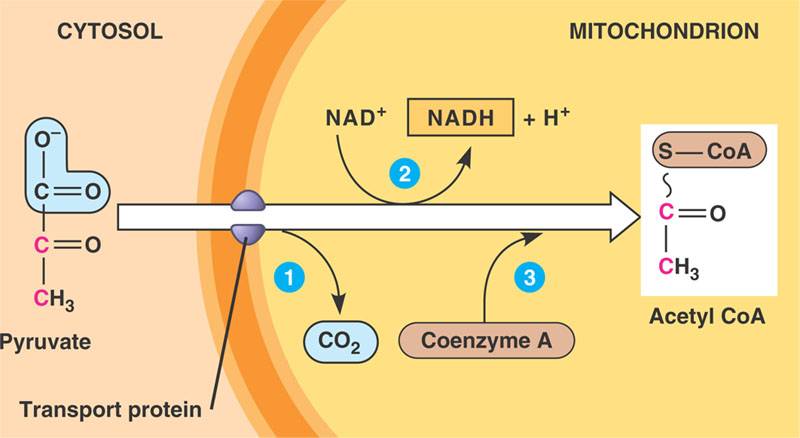
Pyruvate Oxidation
- The product of Glycolysis, (2 Pyruvate molecules) must find a way from the cytosol into the mitochondria matrix to enter the Citric Cycle
- Larger pores in the outer membrane, allow for the diffusion of Pyruvate but a special transport protein is required to move through the inner membrane
- Pyruvate Oxidation commences with a decarboxylation reaction (where is COO- group is lost) This group contain very little energy due to the highly electronegative oxygen group and by removing it allows of the molecule to provide a higher energy output with less energy input
- Then an oxidation reaction transpires afterwards, in which 2 electrons and 1 proton is removed from each Pyruvate group resulting in a reduction to make 2 NADH+ molecules
- Finally the acetyl-CoA reacts with coenzyme A to create the high-energy intermediate to enter the citric cycle
Citric Acid Cycle
- This cycle transpires in the matrix of the mitochondria
- There is the oxidization of acetyl-groups to CO2 as well as the creation of ATP, NADH and FADH
- Per 1 Acetyl-CoA -> 1 ATP (substrate-level phosphorylation), 3 NADH, 1 FADH2, 2CO2, 2H20
- ***O2 is required for this process
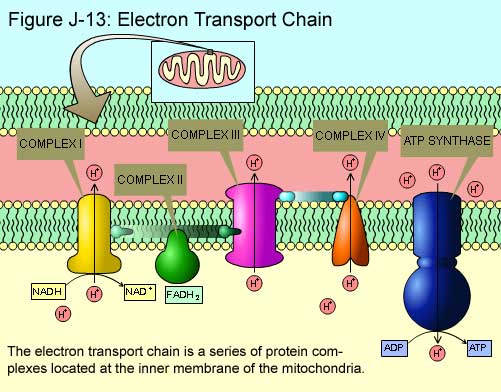
Electron Transport & Chemiosmosis
- After the citric cycle, ALL the carbon in the glucose has been completely oxidized
- There have been the substrate phosphorylation of ATP and potential energy from glucose has been moved into NADH & FADH2
- The purpose of Chemiosmosis is to extract this energy now from NADH and FADH2
- The ETC is found in the inner mitochondrial membrane; facilitates the transfer of electrons from NADH/ FADH2 to Oxygen. (Oxygen is required for this process)
- Complex I: NADH Dehydrogenase; now oxidizes NADH -> NAD+, freeing up one proton (H+) to move into the inner membrane space and two electrons (e-) to proceed along the membrane
- Complex II: Succinate Dehydrogenase; Where FADH2 -> FAD+, freeing up electron and one proton
- Complex III: Cytochrome Complex
- Complex IV: Cytochrome Oxidase; the final complex in the chain before the ATP synthase; Oxygen accepts the electron pair and water is made as a byproduct
- Ubiquinone: Hydrophobic molecule that moves electrons from Complex I to Complex II
- Cytochrome C: Moves electrons from Complex III to Complex IV
- Each of the complexes I, III, IV contain prosthetic groups; that have redox cofactors that alternate between being reduced and then oxidized
- They accept the electron from the prosthetic group of the previous complex and then donate it to the prosthetic group of the next complex
- The electron moves along the ETC from high to low free energy; each complex contains prosthetic groups that are more electronegative than the previous and have a greater affinity for electrons
- Due to the moving along the chain from high energy to low energy; energy is given off at each step along the ETC.
- This energy given off is used to move freed protons from NADH and FADH2 into the inner mitochondrial membrane
- This increases the hydrogen concentration and decreases the pH of the intermembrane space compared to the matrix
- This creates an electrochemical gradient; Higher concentration of charged hydrogen molecules on one side of the membrane
- This creates the proton-motive force (PMF); using this force is called Chemiosmosis!
- The creation of ATP relies on oxidative phosphorylation; the oxidation of molecules to indirectly form ATP
Uncoupling
- The synthesis of ATP by ATP synthase is linked to the proton gradient established during ETC
- NOTE: important to grasp that the ETC and the activity of ATP synthase are distinct processes that are not always coupled
- These processes can be uncoupled by any mechanism that prevents an electrochemical gradient from being established; making the intermembrane permeable to protons
- Ionophores act as uncouplers because they act as a channel in which protons can move into the intermembrane and back into the matrix
- These molecules cause the overall pH of increase and inhibit ATP synthesis; hence are very toxic
Efficiency of Cellular Respiration
- For every 2 electrons pumped through the ETC; 10 electrons are pumped into the intermembrane space
- 3-4 H+ needed for ATP to be synthesized; 3 ATP made per one NADH molecules
- 2 molecules of ATP are made per one FADH2 pumped through the ETC
- Usually a total of 36 ATP is made from this process; However 38 ATP can be made if the NADH from Glycolysis II is from the heart or liver cells already in the matrix; instead of staying in the cytosol and having to be shuttled in
- Efficient: ATP (7.0kcal/mol) Total glucose yield: (686kcal/mol) if 32 yield: 32% efficient
Regulation of Cellular Respiration
- If too much ATP is in cytosol; binds to phosphofructokinase and prevent Glycolysis from proceeding
- Also citrate and NADH concentration can prevent ATP synthesis from proceeding by also binding to phosphofructokinase
- The presence of these molecules in excess means that the subsequent reactions of cellular respiration are happening too slowly; perhaps a lack of oxygen
Catabolism of Fats & Proteins
- Trigylcerides– major source of electron for ATP synthesis; hydrolyzed into glycerol & fatty acids; glycerol is used using in Glycolysis and the other fatty acids are split in Acetyl-CoA for Citric Acid Cycle
- Proteins- hydrolyzed to amino acids before of oxidation as Pyruvate or as intermediates that enter citric cycle
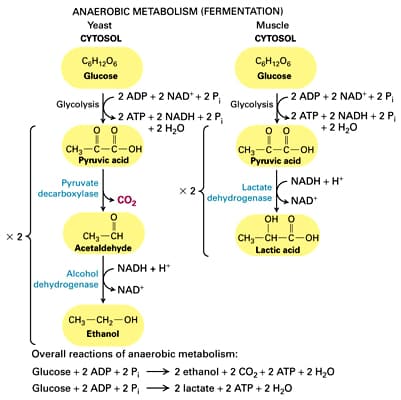
Fermentation
- Occurs when a lack of oxygen is present is present in the cytosol; ATP demand is greater than the amount of oxygen available
- NADH -> is oxidized to NAD+ which is returned to Glycolysis II and is then reduced to make ATP again
- This is an important mechanism for keeping organisms alive during times of reduced oxygen availability (hypoxia)
- Prokaryotes lack mitochondria and therefore must use other methods in order to generate ATP
- However they do have an ETC; some use oxygen as their terminal receptor; others use sulphate, nitrate, ferric ions
- Some prokaryotes are strict anaerobes and require oxygen-free environments to survive
- Some organisms are facultative anaerobes; switch between fermentation and cellular respiration
- Some organisms are strict aerobes: need oxygen at all times to survive (i.e our brain)
Paradox of Aerobic Life
- While oxygen is essential for most life on our planet; it is still quite dangerous to our existence
- When oxygen isn’t fully reduced in the ETC; it is a reactive oxygen species (ROS) (i.e superoxide, hydrogen peroxide) and is a particularly strong oxidizing agent
- They can take electrons from DNA and proteins and cause damage; high levels of these molecules can lead to cell death
- Organisms have developed anti-oxidant defense systems (enzymes & coenzymes)
- Enyzmes such as superoxide dismutase and catalase as well as vitamin C & E help to deal with these ROS
- Oxygen is used as a terminal receptor because it able to pull very large amount of energy from food
- However it should be noted that Cytochrome C holds onto the 4 electrons given to it before it passes them on; therefore the oxygen molecules will be fully reduced to 2 water molecules instead of partially and becoming ROS


very good and simple explanation to complicated process, please also mention about the role of uncoupling proteins in the production of heat, if this heat is not generated and only ATPs generated, it will cause accumulation of energy in the fat and person becomes obese and invites many other problem like diabetes, atherosclerosis, so energy plus heat both are to be generated in balance proportion.
the explanation is very nice it helped me do my group work
Thanks for the lecture, I will also want to know more on biochemistry.