Purpose: To critically explore how osmosis occurs within a semi-permeable membrane with a variety of concentrations of sugar solutions.
Hypothesis: If the 20% concentrated sucrose solution is placed in distilled water, then the weight of the dialysis bag will increase because the fluid within the dialysis bag is hypertonic to the environment. Thus, they will gain water and weight.
Question: How does the weight of the dialysis bag, with a hypertonic solution, change when placed in distilled water?
Observations:
Quantitative:
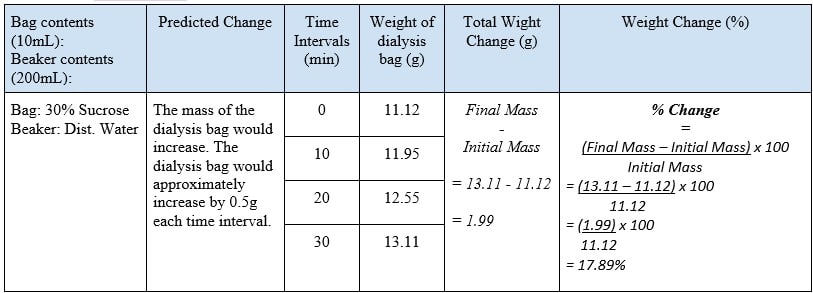
Graph – Supporting Data
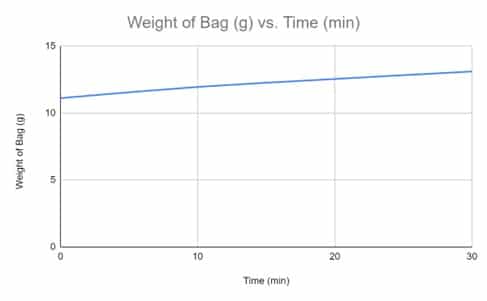
Qualitative (Trends):
Throughout the experiment, a continuous trend was noticed. It was observed that as the time interval increased the weight of the dialysis bag, with 30% concentrated sucrose, would also increase. Although the change of weight per each time interval is not constant, it does not affect the constant relationship between the time intervals and the weight of the dialysis bag because the graph displays the gradual increase of the weight throughout the time intervals. The increase of weight within the dialysis bag occurred due to the fluid within the dialysis bag being hypertonic to the environment, thus, the dialysis bag gained water and weight.
Questions:
Why can’t humans drink salt water for hydration?
Humans cannot drink salt water for hydration because your excretory system would eliminate more water than you have drunk to remove the salt in your body. Also, saltwater has a lower water potential than the water potential of our cells and since water travels from high to low water potential, our cells would shrink; which essentially means that our cells do not have enough water to run our body (also known as dehydration).
Consider what would happen to a red blood cell placed in distilled water.
- Which would have the higher concentration of water?
Distilled water would have a higher concentration of water because it has no solutes, whereas, red blood cells have more solutes than distilled water causing it to have a lower concentration of water than distilled water.
Which would have the higher water potential?
Distilled water would have a higher water potential because it has a low solute potential, meaning fewer solutes are present in this solution. However, in red blood cells, there is more solute potential than in distilled water, hence, red blood cells would have a lower water potential.
Predict what would happen to the red blood cells. Why?
Water travels from high to low concentrations of water; the water then would travel into the red blood cells. Also, the blood cell is placed in a hypotonic solution, meaning more water is diffused in the cell causing the cell to swell and eventually burst.
What is the relationship between a cell’s water potential and the environment’s tonicity (hypertonic, hypotonic, isotonic)?
In a hypertonic environment, a cell’s water potential is higher because water is diffused out of the cell (shrinks the cell). In a hypotonic environment, a cell’s water potential is lower because water is diffused into the cell (swells up the cell). In an isotonic solution, a cell’s water potential is equal to the water potential of the environment because water is equally diffused, in and out of the cell.
How were the results be different when the bags placed in a sucrose solution? Explain the change?
If the dialysis bags were placed in a sucrose solution, the results that we observed would have changed. Our dialysis bag had a 30% sucrose solution; if this bag was placed in a 10% or 20% sucrose solution the weight of the bag would still increase but the total increase would be much less than distilled water because both solutions (in the bag and outside) have some solute potential. However, if the dialysis bag was placed in a 30% sucrose solution the weight would remain the same because both solutions have an equal water potential, hence, water would be diffused equally in and out of the cell.
Conclusion:
If the dialysis bag with a 30% concentrated sucrose solution is placed within distilled water, the weight would increase since more water would diffuse into the cell, this occurs because the solution in the dialysis bag was a hypertonic solution. This lab helped to understand how osmosis occurs within a semi-permeable membrane with a variety of concentrations of sugar solutions. This lab also educated about the different types of solutions and an environment’s tonicity.
