Hess’s Law is an advantageous concept in thermodynamics which states that regardless of the multiple steps or stages in a reaction, the total enthalpy change for a reaction will always be the total sum of all enthalpy changes. The heat created during a combustion reaction is measured by the enthalpy of combustion per mole of the substance burned. Enthalpy itself is the sum of the energy of a system which is calculated by measuring the change in heat of the surroundings. Through this process, the change in heat, therefore energy can be calculated.
+ Qsurroundings = – Qsystem
Calorimetry is a method used to determine the enthalpy change of a chemical reaction by measuring the amount of heat released or absorbed in a reaction. A coffee cup calorimeter is a constant pressure calorimeter and therefore in this device, the heat measured is equivalent to the change in enthalpy. This type of calorimeter is typically used for chemistry based on solutions and generally involves reactions that have very little to no volume change. It is used for low-temperature changes because as the material in a coffee cup calorimeter is made up of styrofoam, the material will melt with high-temperature changes.
A bomb calorimeter on the other hand is the complete opposite in which the volume is constant and gaseous substances can be put in such a device. Furthermore, high changes in temperature can be easily recorded through such a device as well due to the fact that it is made up of a metal material. For both calorimeters, a thermometer would be used to measure the change in temperature.
This experiment aimed to observe and measure the enthalpy changes of two separate reactions: the reaction between magnesium and hydrochloric acid and the reaction between hydrochloric acid and magnesium oxide. A coffee cup calorimeter was used in this experiment to conduct both reactions, which includes a thermometer and a cup that was surrounded by styrofoam and had a stirring rod too. The coffee cup calorimeter was filled with hydrochloric acid where the magnesium oxide and magnesium were added to measure the temperature change. By using the enthalpy changes of these reactions and applying Hess’s Law, the molar enthalpy of the combustion of magnesium was calculated. The enthalpy change of a reaction can be calculated using the equation:
ΔH = q / n.
ΔH represents the change in enthalpy for the reaction, q was the heat absorbed or released and n was the number of moles of the specific substance. Hess’s Law states that the enthalpy change of the reaction was equal to the sum of all the reactions that make up the total reaction.
This experiment’s overall goal was to provide a better understanding of the thermodynamic principles underlying enthalpy changes and combustion reactions. Specifically, to determine the molar enthalpy of combustion of magnesium using calorimetry and Hess’s Law. Finding the enthalpy change by measuring the temperature change of a reaction was also fundamental in the field of thermodynamics. Moreover, the practical applications of Hess’s Law are demonstrated as they may be used in many scenarios such as industrial processes, as although the lab was performed to find the enthalpy changes in magnesium, the process could be used for any element or reaction.
Purpose:
This investigation used Hess’s law to determine the molar enthalpy of the combustion of magnesium, using calorimetry.
Hypothesis:
If the enthalpy of magnesium and magnesium oxide reacting with hydrochloric acid was added with the enthalpy of a hydrogen and oxygen gas reaction to form water in accordance to Hess’s Law (as seen below), then the resulting enthalpy of Mg(s)+½ O2(g)→MgO(s) should be near -618.9 kJ/mol.
This statement was hypothesized by the calculation seen below, using values provided by a previously conducted experiment.

Materials:
- Eye Protection (PPE)
- Steel Wool (for cleaning)
- 1 Electronic Balance
- 1 Thermometer
- 1 Double walled calorimeter
- 100-mL graduated cylinder
- 1 Scoopula
- 0.5g of Magnesium (Mg(s)) in ribbon form
- 1.0g of Magnesium Oxide (MgO(s)) in powder form
- 1.00mol/L hydrochloric acid (HCl(aq))
- 2 100-ml beakers
Procedure:
Cleaned out the calorimeter with a wet paper towel.
- Dried calorimeter with a dry paper towel.
- Measured 100.0ml of 1.00mol/L hydrochloric acid using a 100ml graduated cylinder.
- Poured hydrochloric acid inside the calorimeter chamber.
- Measured and recorded the initial temperature of the 1.00 mol/L solution of HCl(aq) to the nearest 0.2℃ in the calorimeter.
- Added in the 1.00g of MgO(s) to the calorimeter.
- Closed the lid on the calorimeter.
- Stirred the powder vigorously until the solute was dissolved.
- Measured the maximum temperature reading on the thermometer.
- Recorded temperature difference between initial and final maximum temperature
- Properly disposed of the contents of the calorimeter as directed by the teacher.
- Rinsed the calorimeter.
- Dried the calorimeter.
- Collected a 0.5g (to ±0.01g) of Magnesium in ribbon form.
- Polished ribbon with steel wool.
- Repeated steps 2-13 with the 0.5 g of magnesium ribbon.
Observations:
Quantitative observations:
Table 1.1: Temperature of magnesium and magnesium oxide reacting individually with hydrochloric acid
| Reactions With | ||
| Temperature | magnesium metal ribbon | magnesium oxide powder |
| Initial Temperature (℃) | 21.8 | 22.0 |
| Final Temperature (℃) | 40.0 | 28.0 |
Qualitative observations:
Table 2.2: Qualitative observations of magnesium metal ribbon and magnesium oxide powder, reacting individually with hydrochloric acid
| Reactions With | ||
| Observations | magnesium metal ribbon | magnesium oxide powder |
| Visual Observations | – Bubbling seen as contact of both substances was made. – Left a milky white solution after the reaction seemed to be completed. | – Liquid turned cloudy as soon as powder was added to acid. |
| Auditory observations | – Intense fizzing the second the magnesium had made contact with the solution of hydrochloric acid. | None observed. |
| Aromatique observations | – Foul smell was observed upon mixing. | None observed. |
| Taste observations | N/A | N/A |
Results:
Mathematical Calculations & Equations:
Calculate the enthalpy change per mole of magnesium.

Write a thermochemical equation for the reaction of magnesium in acid, including your experimental value.
Mg(s) + 2HCl(aq) → H2(g) + MgCl2(aq) 𝚫H = -428.8kJ
Calculate the enthalpy change per mole of magnesium oxide.

Write a thermochemical equation for the reaction of magnesium oxide in acid, including your experimental value.
MgO(s) + 2HCl(aq) → H2O(l) + MgCl2(aq) 𝚫H = -117.2kJ
Using Hess’s law, the values you have found experimentally, and the given value for the enthalpy change for the formation of water from its elements, determine the molar enthalpy of combustion of magnesium.

Discussion:
Were the changes exothermic or endothermic? Explain.
If the temperature of the surroundings (in this case the solution) increases, it was inferred that energy was lost by the system (the reaction that took place) and that energy was gained by the surroundings (the HCl solution in this experiment). In both reactions, the reactants were approximately at the same temperature before the experiment had commenced. The resulting solution that was made by the addition of Mg(s) or MgO(s) got warmer, hence, the reaction taking place was exothermic because energy was lost from the system and gained by the surroundings. Thus the hypothesis was proven to be true as the reaction did turn out to be exothermic and the energy released was around the number that was predicted (-618.9 KJ). The energy was also “negative” as this refers to the fact that energy was lost from the system, if it was positive this would be an endothermic reaction.
+ Qsurroundings = – Qsystem
Which of the measured values limited the precision of your value? Explain.
The temperature was measured at the approximate highest temperature, which could be a value that was limited in terms of precision. The temperature could change anytime due to a number of errors that could either be human or random such as a warm environment or improper seal of the thermometer to the polystyrene calorimeter. This also includes the lid not being closed fast enough or being opened mid-reaction (to be able to fix the stirring rod in this case), which caused the heat to escape.
Another measured value that could have limited the precision of the end value could be the measurements of the hydrochloric acid, magnesium, and magnesium oxide substances used in the experiment. All of these were measured, although there could have been inaccuracy in the measuring scale, leading to potential systematic error. On top of this, some of the reactants could have been lost, such as the powder magnesium oxide that remained in the beaker and the cleaning of the magnesium strip which could have reduced its weight. This all would have affected the precision of the values.
Explain why (and how) your calculated enthalpies of reaction would be inaccurate if
- Some heat were transferred to the air or Styrofoam cup;
The computed enthalpies of reaction will be incorrect if the heat was lost to the air or a Styrofoam cup while the reaction was taking place. This was due to the measurement of the enthalpy change without taking into account the heat transmission to the environment. An underestimated enthalpy change results from the heat being lost to the environment not being reflected in the heat being transferred to the reaction mixture. Many different processes, including conduction, convection, and radiation, can transfer heat. Convection, for instance, can cause heat to be lost from a process if it occurs in an open container. Similarly, if the reaction happens in a Styrofoam cup, which conducts heat poorly, heat can be lost through conduction.
- The surface of the magnesium ribbon had a coating of MgO(s).
The computed enthalpies of the reaction would be erroneous because the mass of magnesium participating in the reaction would be incorrect if the surface of the magnesium ribbon had a covering of MgO(s). The magnesium ribbon would have additional mass due to the oxide layer on its surface, but this additional mass would not be involved in the process. This would result in an underestimation of the enthalpy of the reaction since the quantity of magnesium that was actually reacting would be less than the starting mass of the ribbon. Also, because magnesium oxide was a stable molecule, it takes more energy to break its bonds than was released when magnesium reacts with hydrochloric acid. Any MgO(s) that might be on the magnesium ribbon’s surface would therefore not be involved in the reaction and would instead absorb some of its heat. The heat absorbed by the MgO(s) would not be taken into account in the calculation, which would lead to an overestimation of the reaction’s enthalpy change. MgO(s) is also heavier than magnesium, hence if the oxygen is removed, the mass of MgO(s) would be less.
Suggest some other possible sources of experimental error in this investigation.
There were various potential sources of error in this experiment. Both human errors and systematic errors were present during the experiment which could have had an impact on the final result. The following errors were observed:
Firstly, it was possible for heat to be lost to the atmosphere or the polystyrene calorimeter while the reaction was taking place. The measured enthalpy value assumed that there was a perfect heat transfer from the system to the surroundings. The heat transported to the reaction mixture does not equal the heat lost to the environment, leading to an underestimation of the enthalpy change. Also, since the reaction takes place in a polystyrene calorimeter, which poorly conducts heat, heat can be lost from the system through conduction.
Secondly, another source of error would be that the magnesium ribbon potentially had extra mass added due to MgO(s) which is heavier than Mg and forms on top of pure exposed Mg like the sample used in this experiment. (The improper cleaning would be a human error) Therefore the computed enthalpies of the reaction could be inaccurate. The additional mass should not be involved in the process and any calculations done using this inaccurate mass value would therefore make the calculations inaccurate.
Also, the lab environment, such as the open windows, dust, and other people conducting the experiment, could have contaminated the equipment, solutions, and metals utilized during the experiment. The magnesium metal’s oxidation, which may have been present since the cleaning process wasn’t precise, was another potential source of inaccuracy. This might change the reaction since there might be a tiny barrier between the magnesium metal and the hydrochloric acid, leading to a false reaction. The reaction was also presumed to be entirely completed, which means that the full change in temperature was presumed to be calculated, but it was also possible that the reaction was not fully completed and the full change in temperature was not measured. Lastly, when transferring the powder to the calorimeter a human error was made in the sense that, while transferring some of the fine granules of the powder flew out in the air and not in the calorimeter. Therefore, again the mass would have been impacted and the calculations could be inaccurate.
Therefore, there were a number of potential sources of error that were presented throughout the experiment.
The accepted value for the molar enthalpy of combustion of magnesium was -601.6kJ/mol. Calculate a percentage difference by comparing your experimental values and the accepted values. Comment on your confidence in the evidence.
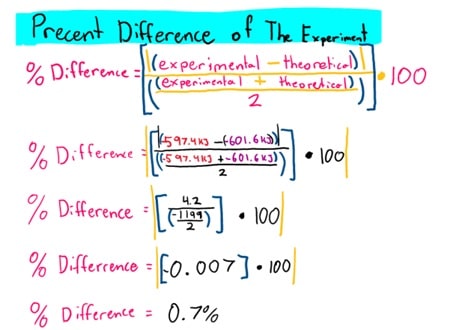
Comment on Confidence in the Evidence: Referring to the minimal sources of error and the percentage difference (calculated above), the result of the final enthalpy value of Mg(s)+½ O2(g)→MgO(s) was highly accurate and further research and calculations are very unlikely to alter the estimates of this value.
Based on your evaluation of the Experimental Design and the evidence, was Hess’s law an acceptable method to calculate enthalpies of reaction?
Absolutely, using Hess’s law to determine reaction enthalpies was an acceptable method. According to Hess’s law, as long as the initial and final circumstances are the same, the enthalpy change of a reaction was independent of the path traveled. It follows that if a reaction can be described as a series of steps, the reaction’s total enthalpy change may be determined by adding the enthalpies of each phase. Hess’s law was founded on the idea that energy can only be moved between objects or changed from one form to another, not created or destroyed. As long as the initial and final states are the same, the overall energy change of a reaction must be the same regardless of the pathway used.
Hess’s law allows for the calculation of enthalpy changes in reactions using experimental data from other reactions with known enthalpy changes. This technique was highly helpful, particularly for reactions that cannot be directly measured experimentally. To calculate the total enthalpy change, it was crucial to ensure that all reactions are legitimate and that the initial and final circumstances are the same.
Suggest an experimental technique that could be used to determine the enthalpy of combustion of magnesium directly.
An experimental method to directly determine the enthalpy of combustion of magnesium in the equation, Mg(s)+½ O2(g)→MgO(s) could be the calculation of enthalpy by utilizing a bomb calorimeter rather than a coffee cup calorimeter as was done in this experiment. A bomb calorimeter would be the perfect tool to use for this experiment as magnesium combusting in air leads to a very exothermic reaction as pure magnesium is a very reactive metal.
A bomb calorimeter is at constant volume and is sealed, thus would be able to better retain the immense amount of heat generated by this reaction. A bomb calorimeter is also specifically designed for combustion reactions as it is able to provide enough energy for the reactants to overcome their activation energy.
The final advantage of using a bomb calorimeter for this reaction would be that since it is made of metal, it will not melt due to the high temperatures compared to a coffee cup calorimeter which would melt. When using a bomb calorimeter, the change in temperature would be measured and with the specific heat of the water and the calorimeter itself, the following equation could be used to determine the enthalpy of the reaction.
q = CΔT
Conclusion:
The purpose of this lab was to calculate the actual molar enthalpy of combustion of magnesium by utilizing Hess’s law and 3 other reactions, one involving Mg(s) and the other involving MgO(s). This final value of molar enthalpy of combustion of magnesium was calculated to be -597.4 kJ through Hess’s law. This value itself was derived from the three reactions occurring throughout this experiment represented through three different equations.
The first equation was the reaction of magnesium and hydrochloric acid to form hydrogen and magnesium chloride, the second equation was the reaction of magnesium oxide and hydrochloric acid to form water and magnesium chloride and the last of hydrogen and oxygen to form water. Using Hess’s Law these three equations were combined to form the target equation of the reaction: magnesium and oxide which forms magnesium oxide. The hypothesis that this entire experiment would expel energy in the form of an exothermic reaction and the resulting value would be around -618.9 kJ was proven to be true as the value of the molar enthalpy was negative and -597.4 kJ. The expected value of enthalpy calculated in the pre-lab was similar to the actual value found (the only difference found was due to the variation in accepted values for specific heat calculations).
Even compared to the accepted value for the molar enthalpy of the combustion of magnesium, the percent difference between that value and the one calculated during the duration of this experiment only varied by 0.7%, further indicating the effectiveness of the procedure carried out. If repeated again, due care must be taken to ensure that the heat does not escape during the duration of this experiment. The lid must be closed on effectively and not left open for extended periods of time. It was also made sure that the magnesium powder or ribbon was measured effectively and not lost during the process of transfer to the calorimeter. Overall, this experiment was successful in determining the value of the molar enthalpy of combustion.
