Introduction:
Fatty acids are long chains of hydrocarbons with COOH groups, or carboxyl groups. Fatty acids are further classified as either saturated or unsaturated based on the type of bonds present. Unsaturated fatty acids contain multiple bonds in addition to single bonds, they are likely to exist as liquids at room temperature. Oils are mixtures of triglycerides, each made up of three fatty acids.
To determine the degree of unsaturation of oils, several methods can be used. In this experiment, the iodine value of values is investigated. By undergoing halogenation of iodine, unsaturated fatty acids will be saturated by breaking one part of the double bonds and forming single bonds with iodine.
Newly synthesized organic compounds will all consist of saturated fatty acids and are called diiodo alkanes, or organoiodine compounds. Iodine value is the number of iodine consumed by fat. A higher iodine value represents a higher degree of unsaturation. Therefore, the problem is what are the iodine values of the oils that are investigated, including olive oil, peanut oil, canola oil, and coconut oil. With these values, the degree of unsaturation of these oils can be determined.
Hypothesis:
It is yet difficult to quantitatively hypothesize the iodine values of the oils. However, rough order of increasing degree of unsaturation can be hypothesized by examining the composition of fatty acids in the oils. The composition of unsaturated fats in olive oil, coconut oil, canola oil, and peanut oil is 86%, 9%, 93%, 80%. Therefore, the order of oils in increasing degree of unsaturation is coconut oil, peanut oil, olive oil, and canola oil. And the order in increasing iodine value maybe coconut oil, peanut oil, olive oil, and canola oil.
Variables:
- Independent: 4 types of oils, olive oil, canola oil, peanut oil, coconut oil, each oil at 1 gm
- Dependent: volume of 0.1 N sodium thiosulfate standard solution titrated into the iodine flasks till the solution turns colorless, iodine value of the oils
- Constants: chemicals (carbon tetrachloride, Wij’s Solution, aka. Iodine chloride, potassium iodide solution, 0.1 N sodium thiosulfate standard solution, 1% starch indicator solution, deionized water), apparatus or equipment (e.g. 250 ml iodine flasks, 50 ml beaker, airtight lids for the iodine flasks, 5 ml pipette, 50 ml burette, burette stand, magnetic stirrer, balance accurate to 4 decimal places, 100 ml graduated cylinder, 20 ml volumetric pipette, 10 ml volumetric pipette, 1 funnel), time the iodine flask is kept in the dark (45 minutes), volume of carbon tetrachloride (20 ml), volume of Wij’s solution (5 ml), volume of potassium iodide (10 ml), volume of deionized water (100 ml), volume of 1% starch indicator solution (5 ml), sign signaling the end of titration (solution turns colorless), rate of stirring of magnetic stirrer (600 rpm), sign signaling the addition of starch solution (solution turns straw color), molar mass of iodine (u) used in calculations
Materials:
- 1 250 ml iodine flask (no uncertainty is included because it is not used to measure volume)
- 1 50 ml beaker
- 1 balance accurate to 4 decimal places, balances accurate to 3 decimal places can also be used
- 4 types of oil with at least 1 g each: olive oil, coconut oil, peanut oil, canola oil
- 1 100 ml graduated cylinder, uncertainty ±0.5 ml
- 1 20 ml volumetric pipette, uncertainty ±0.03 ml
- 1 10 ml volumetric pipette, uncertainty ±0.02 ml
- 260 ml of carbon tetrachloride
- 1 5 ml volumetric pipette ±0.05 ml
- 65 ml Wij’s solution (iodine monochloride)
- 1 airtight lid for the iodine flask
- A stopwatch, accurate to 0.1 s
- 130 ml of potassium iodide
- 1300 ml deionized water
- Magnetic stirrer with a magnet bar
- 1 50 ml burette, uncertainty ±0.02 ml
- 1 burette stand
- 1 funnel
- 650 ml 0.1 N sodium thiosulfate standard solution
- 65 ml 1% starch indicator solution
Procedures:
- One 250 ml iodine flask is labelled “blank,” while the other identical iodine flask is labeled “sample”
- A 50 ml beaker is placed on the balance, the button “tare” is pressed to eliminate the weight of the beaker
- One sample of one type of oil is collected in the beaker with a pipette, for example, olive oil, which should be equal to approximately 0.3 g, though the exact mass is measured by the balance accurate to 4 decimal places and is noted for further analysis
- 20 ml of carbon tetrachloride is measured in the 20 ml volumetric pipette with uncertainty of ±0.03 ml, and added to the beaker
- The beaker is placed on the magnetic stirrer plate, the magnet bar is placed and the magnetic stirrer is turned on, revolving at a rate of 600 rpm
- The solution in the beaker is transferred to the iodine flask, which is placed on the magnetic stirrer plate
- 5 ml of Wij’s solution is measured and then transferred to the iodine flask using a 5 ml volumetric pipette
- The iodine flask is then covered with an airtight lid and kept away in a dark environment for incubation for 45 minutes, a stopwatch is set to 45 minutes countdown
- After 45 minutes, 10 ml of potassium iodide is measured by 10 ml volumetric pipette with an uncertainty of ±0.02 ml and poured into the iodine flask
- The lid is raised, allowing a minimum air gap in order to prevent the formation of iodine vapors as the result of previous reaction
- 100 ml of deionized water is measured by poured into a 100 ml graduated cylinder with uncertainty of ±0.5 ml, then is added to the flask, the magnetic stirrer is kept turned on so that excess iodine is dissolved
- 50 ml of sodium thiosulfate standard solution is titrated into the solution in the iodine flask using a 50 ml burette with uncertainty of ±0.02 ml, which is also used for measuring 50 ml of 0.1N sodium thiosulfate standard solution by pouring the solution into the burette through the funnel placed on top of the burette
- When the solution turned to a straw color, a 5 ml volumetric pipette is used to measure 5 ml 1% starch indicator solution, which is added into the flask, the solution will turn dark blue
- The titration is resumed by adding one drop each time with the magnetic stirrer still on
- Titration is stopped when the solution turns colorless
- The volume of Na2S2O3 titrated is noted
- The procedure above is repeated twice more for olive oil
- Steps 1 to 16 is repeated once without oil added in the first place to obtain the “blank” value of the volume of 0.1 N Na2S2O3 standard solution titrated, this is carried out in the “blank” iodine flask
- Steps 1 to 17 is carried out with other three samples of oil so that altogether 12 sets of data plus the volume of Na2S2O3 solution titrated into the “blank” solution to make the solution colorless will be obtained
Methods of control of variables:
The independent variables are 4 types of oils: olive oil, coconut oil, canola oil and peanut oil added into the 50 ml beaker. Each sample of oil in each trial needs not be equal in weight; however, the exact mass is noted by weighing the oil in a 50 ml beaker on a weighing balance accurate to 4 decimal places.
This is because the weight of oil results in different amounts of titration of sodium thiosulfate solution to make the solution colorless, and the relationship between both is the fundament of investigating the average iodine value of each oil, which will be discussed later.
The dependent variable is the volume of 0.1 N sodium thiosulfate standard solution titrated into the iodine flasks till the solution turns colorless. This is controlled by measuring the difference between the volumes of sodium thiosulfate before and after the titration.
Before the titration, the volume of sodium thiosulfate solution is 50 ml ±0.02 ml, and the volume of the remaining solution is subtracted from the 50 ml ±0.02 ml with an uncertainty of ±0.02 ml of the burette. This volume will lead to the iodine value of the oils, which will be discussed in the next section.
One of the controlled variables is the chemicals used as well as the volume of each chemical used in each trial. Chemicals should be added in the same order and in the same quantity in every trial. 20 ml of carbon tetrachloride, 5 ml Wij’s solution, aka.
Iodine monochloride, 10 ml potassium iodide, 100 ml of deionized water and 1% starch solution are required for each of the thirteen trials. 100 ml graduated cylinder, 20 ml volumetric pipette, 10 ml volumetric pipette, and 5 ml volumetric pipette are used for precise measurement.
Another constant is the equipment for this experiment, including a 250 ml iodine flask, 50 ml beaker, airtight lids for the iodine flasks, 5 ml pipette, 50 ml burette, burette stand, magnetic stirrer, balance accurate to 4 decimal places, 100 ml graduated cylinder, 20 ml volumetric pipette, 10 ml volumetric pipette and a funnel.
If the second piece of equipment is used because there is residue in one container, e.g. there is the residue of Wij’s solution in the pipette but the starch solution is needed to be extracted by the pipette so another pipette is used, the new equipment needs to have the same uncertainty as to the stock container and should be identical to each other.
The next constant is the time the iodine flask is kept in the dark for incubation. The location the flask placed must not have light. The time is measured by a stopwatch which is set for 45 minutes countdown. Once the time runs out, the flask is taken out from the dark environment and can be exposed to light.
The sign signals the addition of starch solution is a constant: when the solution turns straw color after mixing with sodium thiosulfate solution, 1% starch indicator solution should be added.
Similarly, the sign signals the end of titration is also a constant. When the solution turns colorless, which indicates the blue color of iodine reacted with starch disappears because all liberated I2 are bonded with sodium, titration of sodium thiosulfate can be stopped. Though it requires careful observation of the color of the solution.
The rate of stirring of the magnetic stirrer should be kept constant. It is recommended to keep the rate at 600 revolutions per minute or switch to medium if the magnetic stirring plate does not have specific numbers on the revolution rate.
The molar mass of iodine is used in the calculations. It should be constant, e.g. 126.9 u.
Equations and methods for collecting relevant data:
Data table:
| Types of oil | Trial | Mass of oil added (g) | Volume of Na2S2O3 required for “sample” (ml) | Volume of Na2S2O3 required for “blank” (ml) | Normality of Na2S2O3 solution (N) | Molar mass of iodine | Iodine value | Average Iodine value for each oil |
| Olive oil | 1 | 0.1 | ||||||
| 2 | ||||||||
| 3 | ||||||||
| Peanut oil | 1 | |||||||
| 2 | ||||||||
| 3 | ||||||||
| Canola oil | 1 | |||||||
| 2 | ||||||||
| 3 | ||||||||
| Coconut Oil | 1 | |||||||
| 2 | ||||||||
| 3 |
Fatty acids in the oils will react with iodine in iodine monochloride aka. Wij’s solution by halogenation – the addition of the halogen at the C=C double bonds to saturate the oil and produce a di-halogenated single bond, a diiodo alkane, or organoiodine compound, is synthesized, yet it is difficult to name the specific compound.
The solution will turn yellow, which is the same color as iodine monochloride; however, the strength of the color is effectively reduced as much iodine bonds with the precursor alkene (RCH=CHR’) and turns into an organoiodine product (RCHI-CHIR’), which is colorless.
R-CH=CH-R’ + I2 -> R-CHI-CHI-R’
The amount of iodine left not bonded is used to determine the degree of unsaturation of the oil and is determined by adding a solution of potassium iodide to the organoiodine product, which causes the remaining unreacted ICI to separate and iodine will form an iodine molecule.
ICl + KI -> KCl + I2
The molecular iodine I2 shows a straw color, and it is easier to determine its volume with the addition of 1% starch indicator solution which is used as an indicator so that the liberated iodine molecule with react with starch to give a dark blue colored product, so the endpoint can be observed.
The I2 molecule then reacts with 0.1 N standard sodium thiosulfate solution titrated, the product of which will be colorless – meaning that the amount of sodium thiosulfate solution required to make the solution in the iodine flask entirely colorless depends on the amount of I2 molecules in the solutions, which in turn leads to the degree of unsaturation of the oil.
The more sodium thiosulfate required, the more saturated the oil is – which can be further proved that the “blank” solution has the most quantity of sodium thiosulfate titrated due to the fact that all iodine from iodine monochloride is not bonded and all become I2 molecules.
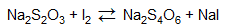
Therefore, the volume of sodium thiosulfate used is the key to the degree of unsaturation. We subtract the volume of Na2S2O4 solution titrated in “sample” from the volume of Na2S2O4 solution titrated in “blank” in order to obtain the difference in the volume of Na2S2O4 solution, which stands for the difference in liberated I2 molecules between two, which differs because more iodine is used in the saturation of the fatty acids in the oils, thus the difference in volume also represents the difference in the degree of unsaturation. The following formula is therefore used.
The iodine value of fat or oil = (Equivalent Wt. of Iodine × (Volume of sodium thiosulfate standard solution used in “blank” titrated (ml) – Volume of sodium thiosulfate standard solution in “sample” titrated (ml)) × Normality of sodium thiosulfate solution (N) × 100 ×10-3) ÷ Weight of fat or oil sample used for analysis (g)
The equivalent Wt. of Iodine should be kept constant in all calculations as discussed. The volume for both amounts of sodium thiosulfate solution titrated in “blank” and “sample” can be acquired by measuring the amount of the Na2S2O4 solution used to make the solution in the iodine flask colorless by looking at the burette reading of the volume of Na2S2O4 solution left in the burette then is subtracted from the original 50 ml of Na2S2O4 solution in the burette in the first place. The normality of sodium thiosulfate solution is 0.1 N by the standard.
The weight of fat or oil sample is weighed at the beginning of the experiment.
Once the iodine value of one trial of one type of oil is determined. The same procedure must be carried out for another two repeats. The average iodine value is thus calculated. At last, compare the average iodine value for all 4 types of oils, an order of degree of unsaturation of these 4 types of oil can be obtained.
Works Cited
“Vegetable Oils Determining Degree Of Unsaturation And Viscosity Biology Essay.”UKEssays. UKEssays, 23 Mar. 2015. Web. 01 May 2017.
” Estimation of Iodine Value of Fats and Oils:.” Estimation of Iodine Value of Fats and Oils (Procedure) : Biochemistry Virtual Lab I : Biotechnology and Biomedical Engineering : Amrita Vishwa Vidyapeetham Virtual Lab. N.p., n.d. Web. 01 May 2017.
“Iodine Value.” Wikipedia. Wikimedia Foundation, 12 Apr. 2017. Web. 01 May 2017.

what is the iodine value obtained for the oils used?
Hey!….the equation between sodium thiosulphate and iodine is not balanced and i was wondering if you could explain using first principles how you get to the formula you have.Am working on a project and your information has been of great help .Thank you .
Hey……thank you for the information but you sort of failed the equation between iodine and sodium thiosulphate……..the products are wrong.please correct it .thank you
Thanks Iwande, the equation has been corrected!