Historically, the term smog referred to a mixture of smoke and fog, hence the name smog. The industrial revolution has been the central cause for the increase in pollutants in the atmosphere over the last three centuries.
Before 1950, the majority of this pollution was created from the burning of coal for energy generation, space heating, cooking, and transportation. Under the right conditions, the smoke and sulfur dioxide produced from the burning of coal can combine with fog to create industrial smog.
In high concentrations, industrial smog can be extremely toxic to humans and other living organisms. London is world famous for its episodes of industrial smog. The most famous London smog event occurred in December, 1952 when five days of calm foggy weather created a toxic atmosphere that claimed about 4000 human lives.
Today, the use of other fossil fuels, nuclear power, and hydroelectricity instead of coal has greatly reduced the occurrence of industrial smog. However, the burning of fossil fuels like gasoline can create another atmospheric pollution problem known as photochemical smog.
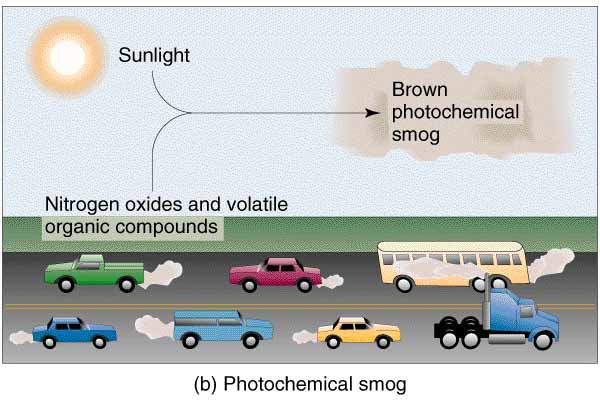
Photochemical smog is a condition that develops when primary pollutants (oxides of nitrogen and volatile organic compounds created from fossil fuel combustion) interact under the influence of sunlight to produce a mixture of hundreds of different and hazardous chemicals known as secondary pollutants.
The development of photochemical smog is typically associated with specific climatic conditions and centers of high population density. Cities like Los Angeles, New York, Sydney, and Vancouver frequently suffer episodes of photochemical smog.
One way in which the production of photochemical smog is initiated is through the photochemical reaction of nitrogen dioxide (NO2) to form ozone. There are many sources of photochemical smog, including vehicle engines (the number one cause of photochemical smog), industrial emissions, and area sources (the loss of vapors from small areas such as a local service station, surface coatings and thinners, and natural gas leakage).
Vehicle engines, which are extremely numerous in all parts of the world, do not completely burn the petroleum they use as fuel. This produces nitrogen dioxide which is released through the vehicle exhaust along with a high concentration of hydrocarbons.
The absorption of solar radiation by the nitrogen dioxide results in the formation of ozone (O3). Ozone reacts with many different hydrocarbons to produce a brownish-yellow gaseous cloud which may contain numerous chemical compounds, the combination of which, we call photochemical smog.
Both types of smog can greatly reduce visibility. Even more importantly, they pose a serious threat to our health. They form as a result of extremely high concentrations of pollutants that are trapped near the surface by a temperature inversion. Many of the components which make up these smogs are not only respiratory irritants but are also known carcinogens.
There are many conditions for the development of photochemical smog:
1. A source of nitrogen oxides and volatile organic compounds.
2. The time of day is a very important factor in the amount of photochemical smog present.
• Early morning traffic increases the emissions of both nitrogen oxides (NOx) and Peroxyacetyl Nitrates (PAN) as people drive to work.
• Later in the morning, traffic dies down and the nitrogen oxides and volatile organic compounds begin to react forming nitrogen dioxide, increasing its concentration.
• As the sunlight becomes more intense later in the day, nitrogen dioxide is broken down and its by-products form increasing concentrations of ozone.
• At the same time, some of the nitrogen dioxide can react with the volatile organic compounds (VOCs) to produce toxic chemicals.
• As the sun goes down, the production of ozone is halted. The ozone that remains in the atmosphere is then consumed by several different reactions.
3. Several meteorological factors can influence the information of photochemical smog. These conditions include :
• Precipitation can alleviate photochemical smog as the pollutants are washed out of the atmosphere with rainfall.
• Winds can blow photochemical smog away replacing it with fresh air. However, problems may arise in distant areas that receive pollution.
• Temperature inversions can enhance the severity of a photochemical smog episode. Normally, during the day the air near the surface is heated and as it warms it rises, carrying the pollutants with it to higher elevations. However, if a temperature inversion develops pollutants can be trapped near the Earth’s surface. Temperature inversions cause the reduction of atmospheric mixing and therefore reduce the vertical dispersion of pollutants. Inversions can last from a few days to several weeks.
4. Topography is another important factor influencing how severe a smog event can become. Communities situated in valleys are more susceptible to photochemical smog because hills and mountains surrounding them tend to reduce the airflow, allowing for pollutant concentrations to rise. In addition, valleys are sensitive to photochemical smog because relatively strong temperature inversions can frequently develop in these areas.
Possible Solutions
A possible solution to the problem of photochemical smog is to enforce stricter emission laws all over the globe. Many countries have varying laws on the legal limits of NOx, Carbon Dioxide, and Sulfur Dioxide.
For example, the United States has a lower legal limit for CO2 than Mexico, which is just south of the U.S. My point is that you can go from one country to another, and notice the differences between the two levels of photochemical smog. If the world were to enforce the same legal smog levels, we wouldn’t have to worry about concentrations of smog in some places more than others.
Another possible solution is to come up with a cleaner-burning fuel for automobiles. Some cars already are being experimented with running hydrogen, electricity, solar power, and even water.
The problem is that these automobiles are not in mass production, therefore, leaving the world to rely on gasoline/diesel as the primary source for power. If the world were to accept the hydrogen car or electric car more openly and develop them for mass production, we would have lower levels of the photochemical pollutants altogether
Photochemical smog can be a significant pollution problem in the Okanagan Valley. The Okanagan meets all the requirements necessary for the production of photochemical smog, especially during the summer months.
During this time period, there is an abundance of sunlight, temperatures are very warm, and temperature inversions are common and can last for many days. The Okanagan Valley also has some very significant sources of nitrogen oxides and volatile organic compounds, including:
1. High emissions of nitrogen oxides and volatile organic compounds primarily from burning fossil fuels in various forms of transportation.
2. The release of large amounts of nitrogen oxides and volatile organic compounds into the atmosphere from forestry and agriculture. Forestry contributes to the creation of photochemical smog creation in two ways: the burning of slash from logging; and, the burning of woodchip wastes in wood product processing plants. Agriculture produces these chemicals through the burning of prunings and other organic wastes.
The idea that the Okanagan is immune to the big city problems of photochemical smog may simply be wishful thinking. In fact, recent monitoring of ground-level ozone has shown that the values between here and the Lower Mainland are quite comparable. In addition, research over a 4 year period (1985-1989) has shown that ozone levels can at times be higher over the Okanagan Valley than the Lower Mainland of British Columbia by almost 49 %.
The Perth Photochemical Smog Study, a joint effort of Western Power Corporation and the Department of Environmental Protection (DEP), was undertaken to determine, for the first time, the extent to which photochemical smog had become a problem in Perth.
Measurements of photochemical smog in Perth’s air began in 1989, at a single site in the suburb of Caversham, 15 kilometers north-east of the city center. Despite the common perception that Perth is a windy city and therefore not prone to air pollution, the first summer of measurements revealed that the city was sometimes subjected to smog levels which approached or exceeded the guidelines recommended by the National Health and Medical Research Council of Australia (NHMRC).
In 1991 the State Energy Commission of Western Australia (SECWA, now Western Power Corporation) sought to extend the capacity of the gas turbine power station it operated at Pinjar, some 40 kilometers north of the Perth central business district. In view of the Caversham data, the Environmental Protection Authority expressed concern that increasing the NOx emissions at Pinjar could contribute to Perth’s emerging photochemical smog problem which, at that stage, was poorly defined.
A consequent condition on the development at Pinjar was that SECWA undertakes a study of the formation and distribution of photochemical smog in Perth, a particular outcome of which would be to determine the effect of the Pinjar power station’s emissions on smog in the region.
Given the DEP’s concerns and responsibility in relation to urban air quality, the Perth Photochemical Smog Study (PPSS) was developed as a jointly operated and managed project, funded by SECWA and with DEP contributing facilities and scientific expertise.
The primary objective of the Perth Photochemical Smog Study was to measure, for the first time, the magnitude and distribution of photochemical smog concentrations experienced in the Perth region and to assess these against Australian and international standards, with consideration given to health and other environmental effects.
The study’s monitoring and data analysis program was very successful in defining the distribution of Perth’s smog. The Perth region experiences photochemical smog during the warmer months of each year. On average, during the three-year period July 1992 to June 1995, there have been 10 days per year on which the peak hourly ozone concentration exceeded 80 parts per billion (ppb) somewhere over the Perth region.
Bibliography
1. Cope, M.C. and Ischtwan, J., 1995, “Perth Photochemical Smog Study, Airshed Modelling Component”, EPA of Victoria, August 1995.
2. Minderly, Calvin 1995, “Photochemical Smog and the Okanagan Valley”, Okanagan University Publishings, June 7-8, 1995.
3. Pidwirny, Michael, Gow, Tracy, et al. “Photochemical Smog”, Microsoft Encarta 1996 Multimedia Encyclopedia. Microsoft Corporation, 1996.
4. Woodward, A.J., Calder, I., McMichael, A.J., Pisaniello, D., Scicchitano, R., Steer, K. and Guest, C.S., 1996, “Options for Revised Air Quality Goals for Ozone (Photochemical Oxidants)”, Project Report to the British Commonwealth Department of Health, Housing and Community Services, August 1993.



I can say two words for this website, awesome and amazing.
Thanks! Really helped me learn
That citation is APA format right?
Btw great website!!
This is an awesome website! Helped me with my assignment and research 🙂
Best website ever! You even had the bibliography for me!
Nice work 🙂