Purpose: Find crystallization temperatures for 7 concentrations of KNO3 and make a solubility graph
Materials: KNO3, test tube, stir rod, weigh boats, hot plates, thermometer, 10 mL graduated cylinder.
Procedure:
-In two test tubes, put exactly 5 mL water in each
-Put assigned amount KNO3 in each
-Heat in hot water bath until dissolved- you may stir with sitr rod
-Find crystallization temperature as test tube cools -use a cold water bath for lower concentrations
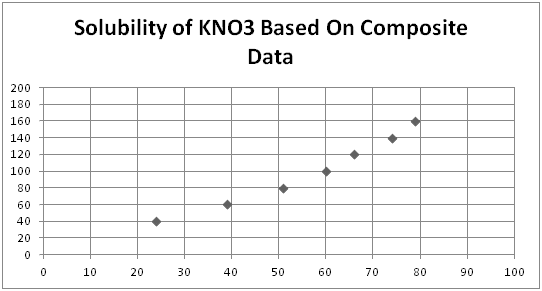
Table:
| Grams KNO3 | Grams H2O | Grams KNO3 per 100g H2O | Actual | Crystallization Temp. | Class Average |
| 2 | 5 | 40 | 24 | 25, 24, 24 | 24, 26, 23, 24 |
| 3 | 5 | 60 | 38 | 41, 40, 33, 46 | 42, 37, 38, 40 |
| 4 | 5 | 80 | 49 | 55, 50, 55 | 49, 51, 50, 53 |
| 5 | 5 | 100 | 57 | 57, 66, 64 | 59, 57, 62, 62 |
| 6 | 5 | 120 | 66 | 71, 71, 64 | 66, 66, 64, 72 |
| 7 | 5 | 140 | 73 | 77, 70, 71 | 73, 77, 72, 73 |
| 8 | 5 | 160 | 78 | 79, 77, 80 | 81, 80, 75, 79 |

Determination of the solubility curve of potassium nitrate questions
Does it have to be cm h2o or grams