Energy
- Capacity to do work
- Kinetic
- Energy possessed by an object because it is in motion
- Potential/Chemical Potential
- Stored energy: the energy an object has because of its location or chemical structure
- Study of energy and its transformations
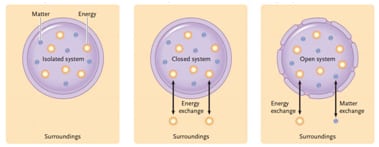
- System
- Object being studied
- Three Types
- Isolated System
- Does not exchanged matter or energy with surroundings
- Ex. Insulated thermos bottle
- Closed System
- Can exchange energy but not matter with surroundings
- Ex. Earth
- Open System
- Both matter and energy can move freely between system and surroundings
- Ex. Anything living
- Isolated System
- Surroundings
- Everything outside the system
- Energy can be transformed from one form into another or transferred from one place to another, but it cannot be created or destroyed
- Ex. Niagara Falls
- Energy transformations are not always efficient
- Energy is lost and unable to do work
- Lost in the form of heat (random molecular motion)
- Every energy transformation increases the disorder/randomness of the universe (Entropy)
- Disorder of an isolated system always increase (entropy)
- Physical objects always break down
- Proteins always break down
- Quality of all life: highly ordered
- Ex. DNA helix, protein, ribosome
- Living cells = open systems
- Exchanging energy and matter with surroundings and use that to generate order
- Eat food to maintain low entropy
- Living things give off heat and carbon dioxide (obeys 2nd law by increasing order)
- No input of energy is required
- Products have lower potential energy than the reactants
- Potential Energy = Enthalpy (H):
- ΔH = Hfinal – Hinitial
- Exothermic: -ΔH
- Release energy
- Endothermic: +ΔH
- Absorb energy
- Products are less ordered than the reactants
- Products have greater entropy
- Entropy (S) increases
- Portion of a system’s energy that is available to do work
Thermodynamics
First Law of Thermodynamics
- Energy can be transformed from one form into another or transferred from one place to another, but it cannot be created or destroyed
- Ex. Niagara Falls
Second Law of Thermodynamics
- Energy transformations are not always efficient
- Energy is lost and unable to do work
- Lost in the form of heat (random molecular motion)
- Every energy transformation increases the disorder/randomness of the universe (Entropy)
- Disorder of an isolated system always increase (entropy)
- Physical objects always break down
- Proteins always break down
Life & Second Law of Thermodynamics
- Quality of all life: highly ordered
- Ex. DNA helix, protein, ribosome
- Living cells = open systems
- Exchanging energy and matter with surroundings and use that to generate order
- Eat food to maintain low entropy
- Living things give off heat and carbon dioxide (obeys 2nd law by increasing order)
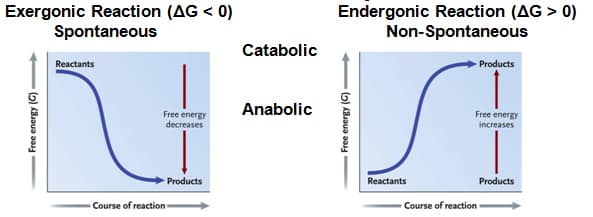
Spontaneous Reactions
Gibbs Free Energy
∆G = ∆H – T∆S
∆ G = G final state – G starting state
Contributions of Enthalpy & Entropy
- High Free Energy

- Less stable
- Greater work capacity
- Systems spontaneously change into more stable state
- Ex. Glucose into carbon dioxide
- Ex. Concentration gradient
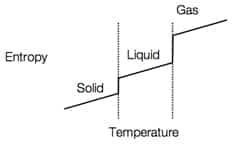
- Transformation from solid > liquid > gas allows for an increase in entropy
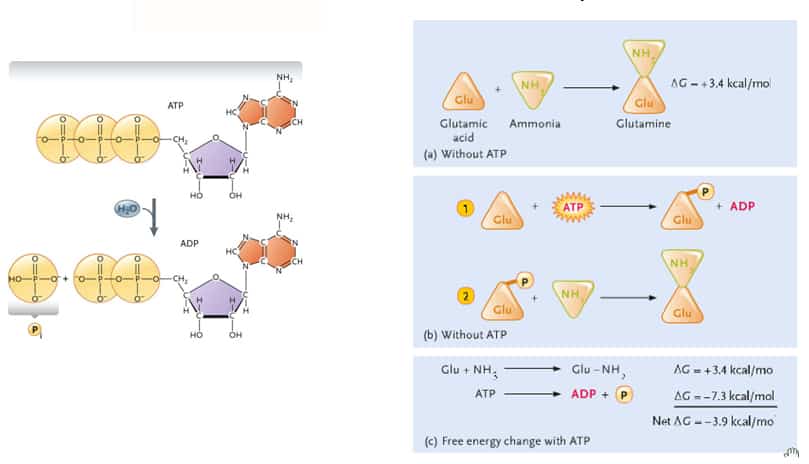
Adenosine Triphosphate
- Contains large amounts of free energy due to high-energy phosphate bonds
- Phosphate groups closely associated with each other – negative charges repel = bonding arrangement unstable
- Removal of phosphate group – spontaneous reaction – releases large amounts of free energy
- Hydrolysis reaction = warming reaction – DOES NOT OCCUR (RARELY) IN CELLS
- Energy Coupling
- ATP is brought into close contact with reactant group
- Terminal phosphate group is transferred to the reactant molecule making it less stable (phosphorylated)
- High phosphoryl group transfer potential
- Enzyme mediated (active site does not bind to water – only ATP and reactant molecule)