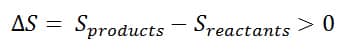The First Law of Thermodynamics: This is the law of conservation of energy. The total amount of energy in the universe is constant. Energy cannot be created nor destroyed.
We have seen that energy can be transferred from a system to its surroundings and vice versa. During these processes no energy is created nor destroyed
Enthalpy Changes: Energy is used to break bonds, and energy is then released when new bonds are formed. The difference between these energies is the enthalpy change.
Bond Energy: The energy required to break a bond. High energies correlate to very stable bonds.
| Bond Type | Bond Energy
(kJ / mol) |
| H – H | 436 |
| C – H | 413 |
| N – H | 391 |
| C – C | 346 |
| C = C | 615 |
| C – N | 305 |
| O – H | 436 |
| C – O | 358 |
| C = O | 749 |
| N – O | 222 |
Spontaneous Reactions: Reactions that occur naturally and do not have to be forced to occur (by using heat or electricity).
Non-spontaneous reactions: reactions that do not occur unless energy is put in to force them to occur.
Exothermic reactions are often spontaneous. Eg: combustion reactions.
Endothermic reactions are often non-spontaneous Eg: electrolysis and decomposition
However, many endothermic processes are spontaneous
Eg: dissolving ammonium nitrate in water absorbs heat.
Why do reactions yielding less stable products occur spontaneously?
Entropy (S): The measure of disorder or randomness. It can apply to a system, surroundings, or the universe as a whole.
Eg: A deck of cards laid out with all the suits grouped up and in order from ace to king is very ordered. This would have a low entropy. A deck of cards thrown in the air would land randomly on the floor with all the cards mixed up. This would have high entropy.
Second Law of Thermodynamics: The entropy of the universe is always increasing.
- This means that all processes tend to be spontaneous if they increase in entropy
- Processes that decrease entropy are possible only if they are accompanied by an equal or greater increase in the entropy of the universe.
Eg: People building things must break down glucose and fats for energy.
- Spontaneous processes tend to have increases in entropy (meaning more disorder)
Reaction Entropy: ΔS > 0 if;
- There are more moles of product made than moles of reactant used
- Complex molecules are broken into simpler subunits
- A substance changes state from solid to liquid/gas or from liquid to gas
Gibbs Free Energy
Gibbs FreeEnergy (G): The amount of energy that is available to do work. This can be found by combining the enthalpy and entropy of a system.
Spontaneity: The spontaneity of a process depends on whether or not the change in Gibbs free energy is negative (ΔG<0).
ΔG < 0 reaction is spontaneous
ΔG > 0 reaction is not spontaneous