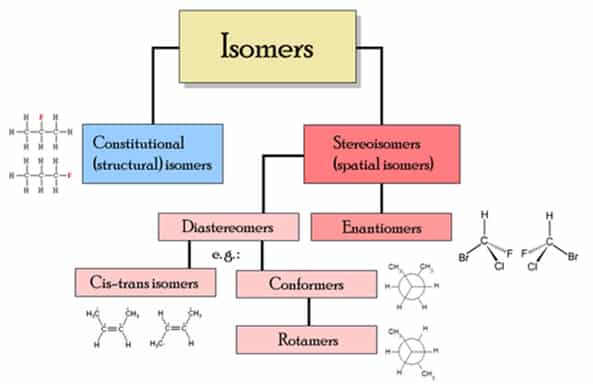
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. The word is derived from the Greek ισομερης, isomerès; isos = “equal”, méros = “part”. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical isomers (see chart below). Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are two main forms of isomerism: structural isomerism and stereoisomerism (spatial isomerism).
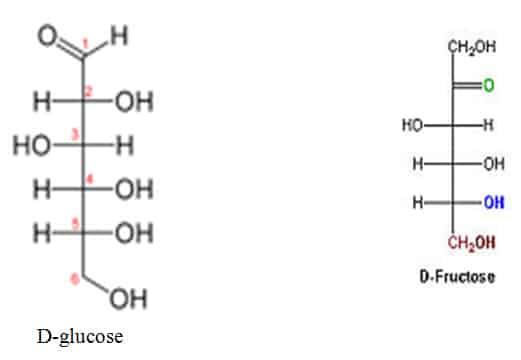
Glucose and Fructose are Diastereomers
Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other.[
D-glucose
High–fructose corn syrup, however, refers to a family of mixtures of varying amounts of glucose being converted into fructose and addition of the corn syrup for the sweet taste.
Yeast enzymes convert sugar (glucose, or fructose) to ethanol and carbon dioxide. However, it is the 5-ring form of fructose that is sweeter;