Background
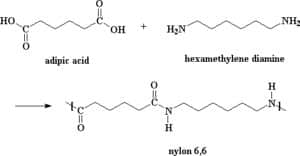
Nylon is a polyamide that is formed from the condensation reaction of adipic acid (a dicarboxylic acid) and 1,6 – diaminohexane (a diamine). A scientist working for DuPont, Wallace Carothers discovered Nylon in 1935 and its name is a contraction of New York and London. When Nylon was first discovered it was used to make many war materials such as parachutes, ropes and army boot laces (van Kessel, H. et al., 2003).
However, nowadays Nylon is used in many other products such as clothing, women’s stockings, climbing equipment, thick toothbrush bristles and even bulletproof vests. Nylon’s use in many different products is due to its, “light weight, strength, durability, and resistance to damage” (Smith, 2009).
In addition, it’s ability to take dye means that in can be made into many different colours, which is important for clothing manufacturers (Smith 2009). Many companies benefit from the discovery of Nylon because it is less expensive to produce clothing using Nylon and the clothing manufactured can be much better quality than other materials.
Dangers
Unfortunately, although Nylon itself does not contain any compounds that are dangerous to the environment or one’s health, manufacturing Nylon does. The process of manufacturing Nylon releases nitrous oxides and since factories have no use for the byproduct, it is released into the atmosphere as waste.
It has been discovered that, “nitrous oxides contribute to the destruction of stratospheric ozone and is a powerful greenhouse gas” (Nylon: sheer havoc […], 2004). This shows that although humans have benefited from the discovery of Nylon, its production is harmful to environment, especially in mass quantities.
The same article also stated that, “[…] scientists calculate that Nylon production accounts for one-tenth of the annual 0.2 percent increase in atmospheric levels of the gas ” (Nylon: sheer havoc […], 2004). This is a very large increase due to the production of Nylon for one year, particularly considering that, “[…] nitrous oxide has an atmospheric lifetime of one-hundred and fifty years” (Nylon: sheer havoc […], 2004).
From this one can understand that nitrous oxide accumulates in the atmosphere and assists in the destruction of the ozone (Nylon: sheer havoc […], 2004). In addition, if production of Nylon began roughly around 1935 one can assume that due to the atmospheric life of nitrous oxide all the fumes released into the air since 1935 are still in the lower stratosphere destroying our ozone layer.
Another problem with the production of Nylon is the health concerns related to its thermal processing. Thermal processing of Nylon can cause many problems if one is exposed to the fumes or dust. Some of these problems include irritation of mucous membranes in the nose and throat, mechanical irritation of the eye and irritation of the skin.
As well, if working with the polyamide before it is cooled workers can have their skin burned. Thirdly, if anyone were to ingest/inhale they may experience gastrointestinal discomfort (Malloy and Grubb, 2008). These are the health concerns that face workers in closer proximity to the production of Nylon.
Disposal
In continuation, after Nylon products are used and are no longer wanted they are thrown in the garbage and therefore mankind must also have a method to dispose of Nylon. This causes a problem because Nylon has an exceptionally slow decay rate, which means that there is a buildup of Nylon products in landfills worldwide (Smith 2009).
Therefore, an alternative method of disposing Nylon is to incinerate it, however this method has some harmful products. The most common products of Nylon’s thermal breakdown include, “carbon monoxide, ammonia, aliphatic amines, ketones, nitriles and hydrogen cyanide” (Malloy and Grubb, 2008).
However, some products may differ depending on various factors such as temperature, time of exposure and environmental factors (Malloy and Grubb, 2008). These products are not good for the environment or our health because for example, hydrogen cyanide, which is a gas just above room temperature, is highly poisonous (Hydrogen Cyanide, 2009).
After reading through the risks and benefits of making Nylon products, one can understand that it is better to use alternative products such as cotton for the production of cotton. However, there are certain products that Nylon should be used for products such as bulletproof vests, parachutes and sporting equipment like rock climbing ropes.
In these cases, Nylon is the right material to be used because of its durability and strength. For example, when polyamides are linked together into stacks they form extremely strong fibers which when put together are very resistant to damage and can even stop a bullet (van Kessel, H. et al., 2003). This is why Nylon is used in part to make bulletproof vests for people such as police officers who come into situations involving guns on occasion.
For products that need to be durable and strong because they are being used in dangerous situations, Nylon should be used, however, it does not need to be used in everyday clothing. Everyday clothing does not need to be extremely durable as most people do not have jobs that require lots of activity, therefore materials such as cotton could be used more commonly.
Although this would be slightly more expensive, it would reduce the amount of nitrous oxides being released into the air and also cut down on the build-up of Nylon waste in landfills.
In conclusion, an idea for the future is, when the technology exists, to have machines and/or robots that do the hands on work of humans in the environments where these gases are. This would significantly reduce the risk workers take every day and also eliminate most of the health concerns as workers would rarely have to come in contact with the fumes or dust created by the production of Nylon.
References
(2009). Hydrogen Cyanide. Retrieved from http://www.britannica.com/
EBchecked/topic/278704/hydrogen-cyanide
(2004). Nylon: sheer havoc – mass production of nylon may be a factor in global
warming. Retrieved from http://findarticles.com/p/articles/mi_m1200/
is_n8_v139/ai_10426323/
Malloy, T., & Grubb, M. (2008). MSDS – Nylon 6. Retrieved from http://skipper.physics
.sunysb.edu/HBD/MSDS/NylonMSDS.pdf
Smith, S. E (2009). What is Nylon?. Retrieved from http://www.wisegeek.com/what-is-
nylon.htm
van Kessel, H., Jenkins, Dr. F., Davies, L., Plumb, D., & Lantz, Dr. O. (Ed.). (2003).
Chemistry 12. Toronto, Canada: Nelson.



Thanks