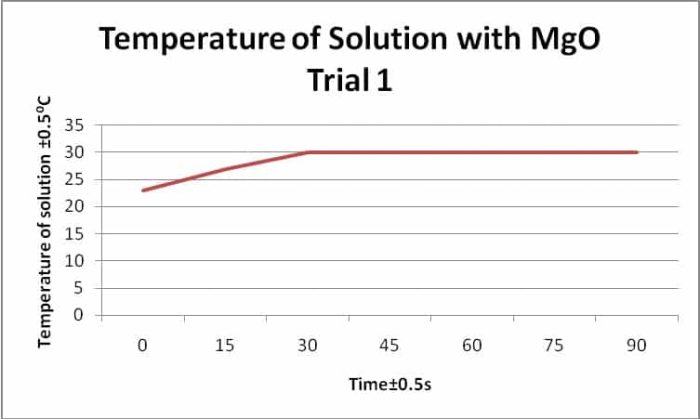
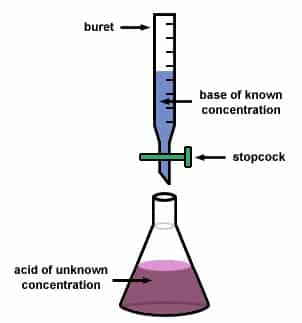
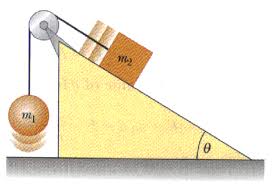
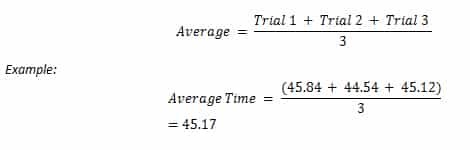Fetal Pig Dissection Lab Answers
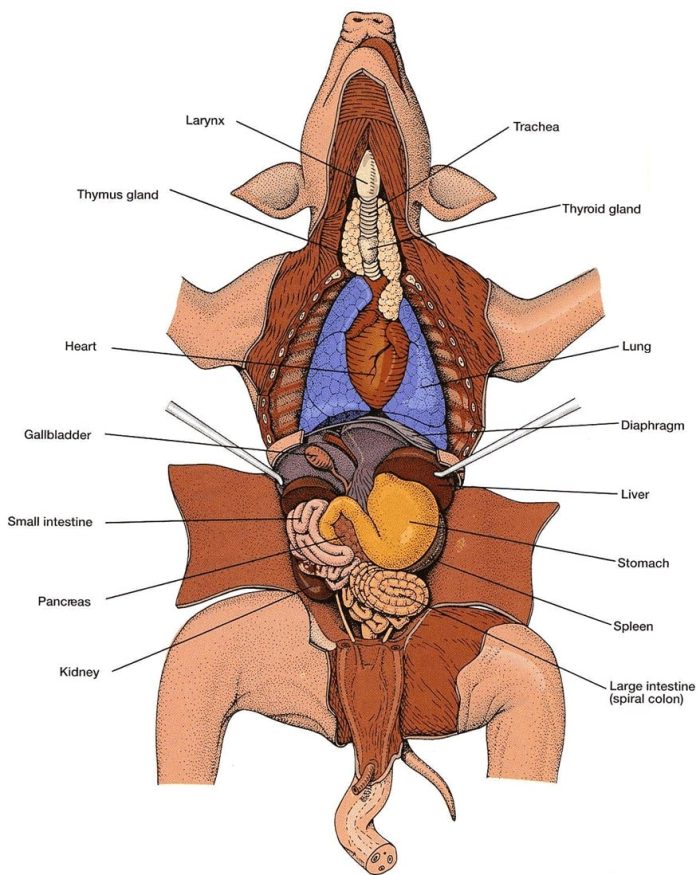
Introduction Pigs, one of the most similar animals to humans, have been used to inform and teach students about the circulatory, respiratory, and digestive systems through a procedure called a dissection for many years. Pigs are similar to humans through the fact that they have skin, not fur or feathers, they are omnivores, and when…