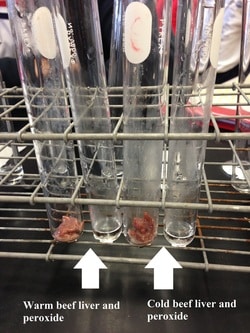
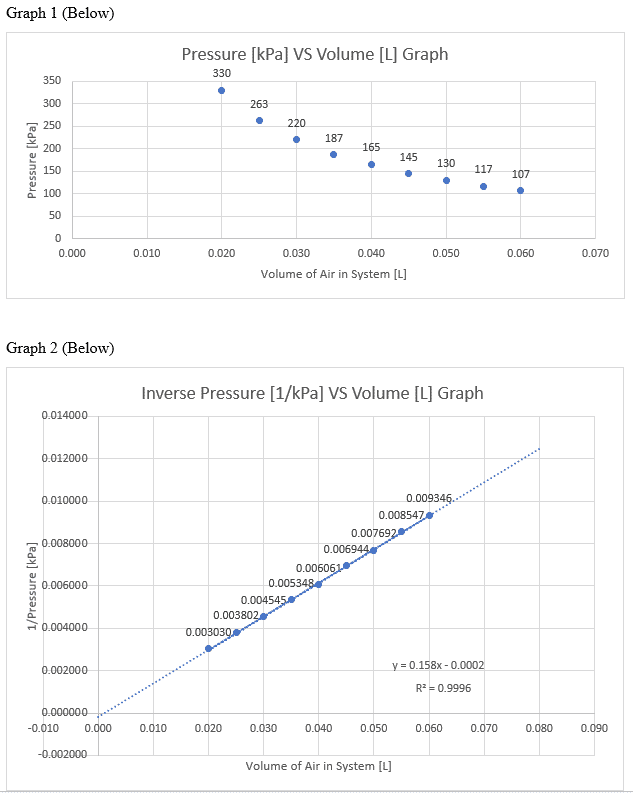
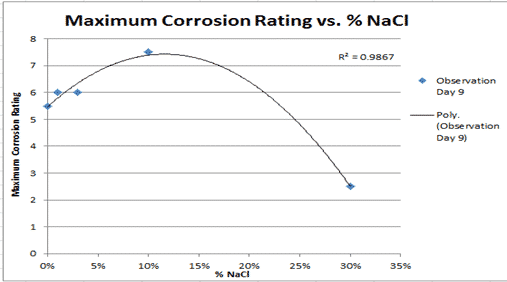
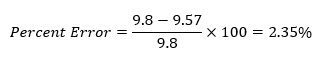The Effect of Temperature on Hydrochloric Acid and Bath Bombs to Produce Carbon Dioxide Lab Answers
INTRODUCTION: Bath bombs consist of a wide range of ingredients, including bath salts, food coloring, fragrance, citric acid, sodium carbonate, and other components. Bath bombs ‘fizz’ when water inclines to trigger a reaction between an acid and a base (neutralizing substance). Many bath bombs contain citric acid, and sodium carbonate, which incline to have a…