The Workplace Hazardous Materials Information System (WHMIS) is a comprehensive national system for safe management of hazardous chemicals, which is legislated by both the federal and provincial jurisdictions.
WHMIS is a three (3) part effort of labour, industry, and government which took several years to develop. It is unique in that it represents a consensus of these three groups. WHMIS legislation provides that workers must be informed about the hazards in the workplace and receive appropriate training to enable them to work safely.
The key elements of the system are:
- Labels and/or Symbols;
- Material Safety Data Sheets (MSDS);
- Worker Education.
WHMIS requires all suppliers to label and prepare Material Safety Data Sheets (MSDS) for products they make, import, package, or process. The ultimate goal is to create a safer workplace by providing workers with the knowledge and tools to enable them to work safely.
Symbols
The WHMIS system groups hazardous materials into eight classes or categories based on the type of hazard which they represent. These materials are also called controlled products. Each category has its own hazard symbol and it is important that the worker be able to recognise these.
- Compressed Gas (A): A compressed gas is a material which is a gas at normal room temperature (20 C) and pressure but is packaged
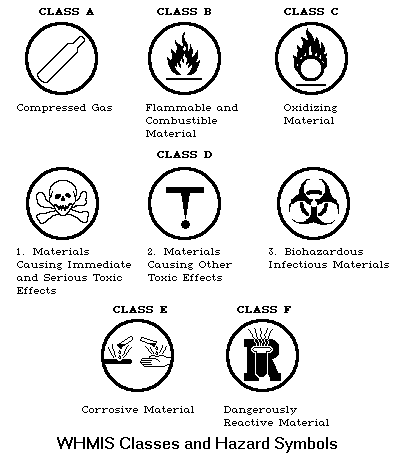 as a pressured gas, dissolved gas or gas liquified by compression or refrigeration. The hazard from these materials, aside from their chemical nature, arises from sudden loss of integrity of the container. A compressed gas cylinder is usually quite heavy and when ruptured can become a projectile with the potential to cause significant damage. Acetylene and oxygen are examples of compressed gases.
as a pressured gas, dissolved gas or gas liquified by compression or refrigeration. The hazard from these materials, aside from their chemical nature, arises from sudden loss of integrity of the container. A compressed gas cylinder is usually quite heavy and when ruptured can become a projectile with the potential to cause significant damage. Acetylene and oxygen are examples of compressed gases. - Flammable and Combustible Material (B): Flammable or combustible materials will ignite and continue to burn if exposed to a flame or source of ignition. Materials are classified as a flammable gas, flammable aerosol, flammable liquid, combustible liquid, flammable solid, or reactive flammable material. Gasoline, engine oil, WD-40, butane, isopropyl alcohol and Varsol are examples of flammable materials.
- Oxidizing Material (C): An oxidizing material may or may not burn itself, but will release oxygen or another oxidizing substance, and thereby causes or contributes to the combustion of another material. Ozone, chlorine, and nitrogen dioxide are oxidizing materials. These chemicals will support a fire and are highly reactive.
- Poisonous (D1): Poisonous materials that cause immediate and serious toxic effects. These materials may be classified as toxic or very toxic (based on information such as LD50 or LC50). Examples: Isopropyl alcohol and hydrogen cyanide are very toxic substances.
- Toxic (D2): Toxic materials that cause other toxic effects. A pure substance or mixture that may be any one of the following: a carcinogen, teratogen, reproductive toxin, respiratory tract sensitizer, irritant or chronic toxic hazard. Examples: Asbestos causes cancer, ammonia is an irritant.
- Biohazardous Infectious Material (D3): This classification includes any organisms and the toxins produced by these organisms that have been shown to cause disease or are believed to cause disease in either humans or animals. For example, a blood sample containing the Hepatitis B virus is a biohazardous infectious material. It may cause hepatitis in persons exposed to it.
- Corrosive Material (E): Corrosive materials can attack (corrode) metals or cause permanent damage to human tissues such as the skin and eyes on contact. Burning, scarring, and blindness may result from skin or eye contact. Corrosive materials may also cause metal containers or structural materials to become weak and eventually leak or collapse. Sulphuric acid, hydrochloric acid, and ammonia are examples of corrosive substances.
- Dangerously Reactive Material (F): Dangerously reactive materials may undergo vigorous polymerization, decomposition or condensation. They may react violently under conditions of shock or an increase in pressure or temperature. They may also react vigorously with water to release a toxic gas. Acetylene is an example of a dangerously reactive material.
Key Terms and Definitions
Biohazardous Infectious Material (D3): Organisms and the toxins produced by these organisms that cause disease in either humans or animals.
Carcinogen: A substance that causes cancer.
Compressed Gas (A): A material that is a gas at normal room temperature and pressure but packaged as a pressured, dissolved or liquefied gas.
Condensation: To change from a gas or vapour to a liquid.
Corrosive Material (E): A material that can attack (corrode) metals or cause permanent damage to human tissues such as the skin and eyes on contact.
Dangerously Reactive Material (F): A material that may undergo vigorous polymerization, decomposition or condensation and may react violently under conditions of shock or an increase in pressure or temperature.
Decomposition: The act of decaying (to putrefy).
Flammable and Combustible Material (B): A material that will ignite and continue to burn if exposed to a flame or source of ignition.
LC50: Lethal concentration: 50% (percent) kills.
LD50: Lethal dose: 50% (percent) kills.
Oxidizing Material (C): A material that may not burn itself, but will release oxygen thereby causing or contributing to the combustion of another material.
Polymerization: The process of changing the molecular arrangement of a compound so as to form new compounds.
Poisonous (D1): A material that causes immediate and serious toxic effects.
Teratogen: The production of abnormal organisms.
Toxic (D2): A material that causes other toxic effects.
