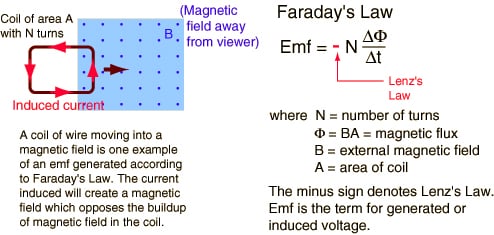
Lenz’s Law and Magnetic Flux
With this definition of the flux being, we can now return to Faraday’s investigations. He found that the magnitude of the emf produced depends on the rate at which the magnetic flux changes. This fundamental result is known as Faraday’s law of induction. The minus sign is placed there to remind us in which direction…