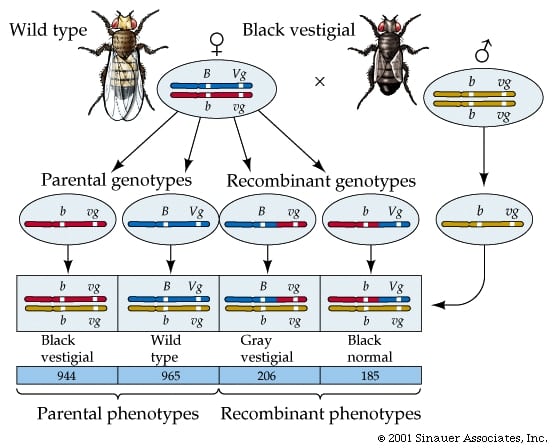
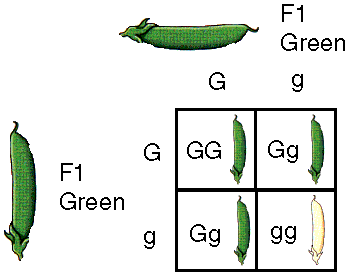
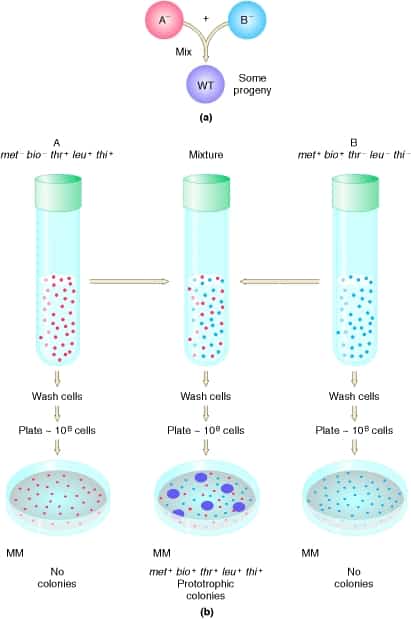
Genetic Recombination in Eukaryotes: Meiosis
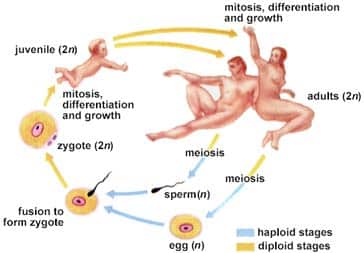
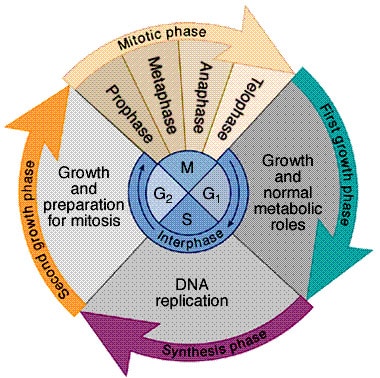
Sexual reproduction: production of offspring; union of male and female gametes Gametes: eggs and sperms cells in animals Sexual reproduction depends on Meiosis; specialized process of cell division; recombines DNA sequences, produces cells with half the number of chromosomes in somatic cells. (chromosome= reduction) Fertilization: nuclei of egg/ sperm fuse; producing zygote (# chromosomes typical…