Topographic Maps
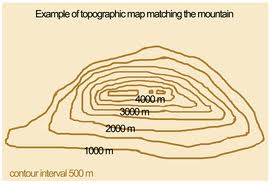
Topographic maps are made specifically to show elevation. They show elevation through the use of contour lines. *The mountain below has 1 peak! 4000m What is the lowest elevation of this mountain? 1000 m PLEASE NOTE: Not every elevation can be represented by a contour line. Index contours: bold contour (lines used to calculate the…