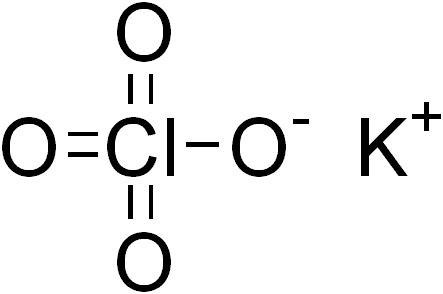William completed his Bachelor of Science and Master of Arts in 2013. He current serves as a lecturer, tutor and freelance writer. In his spare time, he enjoys reading, walking his dog and parasailing.
Article last reviewed: 2022 | St. Rosemary Institution © 2010-2025 | Creative Commons 4.0
Some materials are so commonplace that we take them for granted. One of those materials is a grayish metal that has been with us for thousands of years. That metal is lead, still one of the world’s most useful substances, and one that never ceases to find a role in human society. Lead has the…
Iron in its pure state is soft, malleable and ductile (that can be stretched, drawn or hammered thin without breaking ((Webster’s Dictionary, 419, 1988)) with a hardness of 4-5. It is easily magnetized at room temperatures and this property disappears when heated above 790 degrees Celsius.. Metal iron occurs in a free state in only…
An ideal gas is a theoretical gas that perfectly fits into the equation PV= nRT. An ideal gas is different from a real gas in many ways. Ideal gases abide by all gas laws regardless of the pressure of temperature; however in reality they do not exist, hence the terminology “ideal”. They occupy no volume,…
Production Although Helium is one of the most common elements in the universe it is a rare gas on earth. It exists in the atmosphere in such small quantities (less than five parts per million) that recovering it from the air is uneconomical. Helium is produced as a by-product of the refining of natural gas,…
Gallium, atomic number 31, is very similar to aluminum in its chemical properties. It does not dissolve in nitric acid because of the protective film of gallium oxide that is formed over the surface by the action of the acid. Gallium does however dissolve in other acids, and alkalies. Gallium was discovered (1875) by…
I. Purpose To determine the freezing point of a known substance, naphthalene II. Materials ringstand gas source test tube test tube clamps thermometer naphthalene Bunsen burner goggles hose stopwatch III. Procedure 1. Assemble the Bunsen burner, attaching one end of the hose to the burner and the other to a gas source. 2. Assemble the…
The Frank J. horgan Filtration Plant is located Southeast of Toronto on the shores of Lake Ontario (See map). Its purpose is to provide safe drinking water to our taps by filtering the water. The water is gathered from Lake Ontario. This plant has a production capacity of 455 million litres per day to supply…
Purpose In this lab we will observe the products of decomposition of potassium perchlorate (KClO4). We will then predict from our results the correct chemical reaction equation. Procedure 1. Weigh out about 4.0g of KClO4 in a test tube. Record the accurate weight below. Product Weight Before Weight After Mass of Test tube + KClO4 …
Copper is a mineral. It is not a plant or an animal. Copper is a metallic metal. It can never be broken down into different substances by normal chemical means. Copper was one of the first metals known to humans. People liked it because in its native condition, it could easily be beaten into weapons…
(C18 H2, NO3 H3PO4 1/2 H2O) Codeine is known medically as methylmorphine. It is a drug derived from opium, a poppy plant. It was discovered in 1832 by French chemist Pierre-Jean Robiquet. Codeine constitutes about 0.5 to 2.5 percent of this plant substance. The drug has been in use since the early 1900’s and it…
Cobalt is the 27th element on the periodical table and has an atomic number of twenty-seven. It has a symbol of Co. Cobalt¹s atomic weight is 58.9332. It has a melting point of 1,490š C. and a boiling point of 2,900š C. Cobalt looks almost exactly like iron and nickel. Cobalt is between iron and…
Chlorine is (at room temperature) a greenish-yellow gas that can be readily liquefied at 5170 Tarr or 6.8 atmospheres, at 20 C (68 F), and has a very disagreeable odor. Its Element Symbol is Cl, atomic number is 17, and atomic mass is 35.453. Chlorine’s melting point is -101 C or 149.8 F. The boiling…
Chloroflourocarbons were discovered in the 1920’s by Thomas Midgley, an organic chemist at General Motors Corporation. He was looking for inert, non-toxic, non-flammable compounds with low boiling points that could be used as refrigerants. He found what he was looking for in the form of two compounds: dichlorodifluoromethane (CFC-12) and trichloromonoflouromethane (CFC-11). In both compounds,…
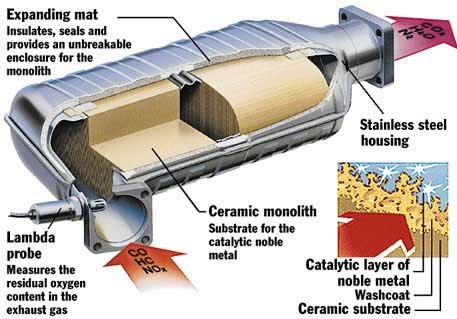
Notice when a vehicle has driven by nowadays, that it is so much quieter than those loud oldies that pour out the blue smoke. Ever wonder just what is underneath a vehicle that makes the new ones so much cleaner. It is called a catalytic converter. The main function of a catalytic converter is to…
Spray cans produce an aerosol, the technical term for a very fine spray. They do this by means of a pressurized propellant, which is a liquid that boils at everyday temperatures. Inside the can, a layer of gaseous pressure increased, and eventually it becomes so high that boiling stops. When the nozzle is pressed, the…
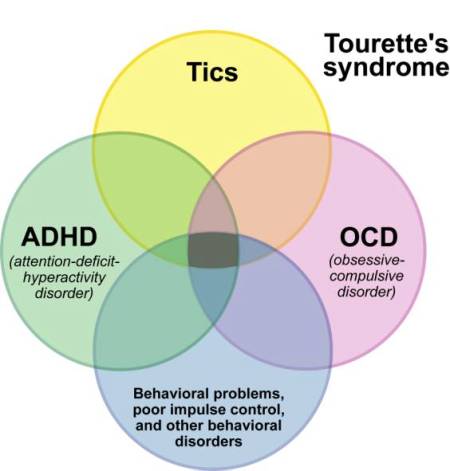
Tourette Syndrome was named for Georges Gilles de la Tourette, who first described the syndrome in 1885. Although the disease was identified in 1885, today in 1996, there still is a mystery surrounding Tourette Syndrome, its causes and possible cures. Tourette Syndrome is a neurological disorder that researchers believe is caused by an abnormal metabolism…
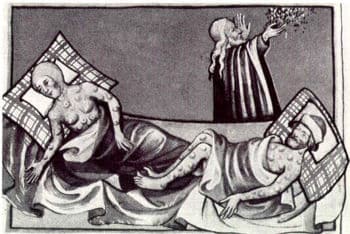
Since the reign of Emperor Justinian in 542 A.D., man has one unwelcome organism along for the ride, Yersinia pestis. This is the bacterium more commonly known as the Black Death, the plague. Plague is divided into three biotypes, each associated with one of three major pandemics occurring in history. Each of these biotypes are…
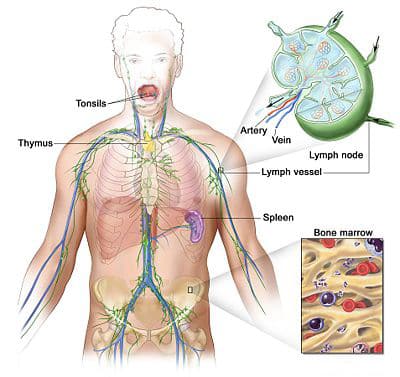
The Lymphatic System is very important. It helps with the Cardiovascular system, and our immune systems. The Lymphatic System is made up of two semi-independent parts. One is a network of lymphatic vessels. The other part is various lymphoid tissues and organs all over the body. The functions of the Lymphatic System transporting fluids that…
The koala is the Australian jewel. It has very furry, ash colored hair, a rubbery black nose, sharp claws, fuzzy ears, and a grizzly personality. If you kill a koala, you’ll make a million off their fur! They would sell the fur to coat companies and make coats out of koala fur. Well, sadly enough,…
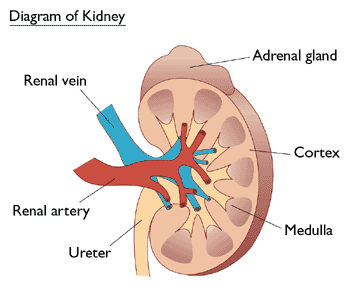
Invertebrates, kidneys are the two major organs of excretion. Excess water, toxic waste products of metabolism such as urea, uric acid, and inorganic salts are disposed of by kidneys in the form of urine. Kidneys are also largely responsible for maintaining the water balance of the body and the pH of the blood. Kidneys play…